Fused ring compounds
a technology of ring compounds and ring compounds, applied in the field of ring compounds, can solve the problems of tumor shrinkage, poor prognosis of ras mutation in cancer,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
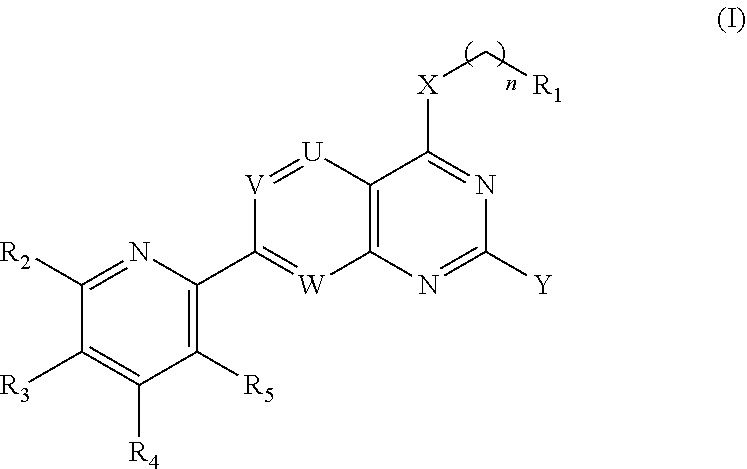
[0398]A compound of Formula (I):
[0399]or a pharmaceutically acceptable salt thereof;[0400]wherein,[0401]R1 is an electrophilic moiety capable of forming a covalent bond with a cysteine residue at position 12 of a K-Ras G12C mutant protein;[0402]R2 is selected from the group consisting of H, OH, NH2, halo, C1-6 alkyl, C1-6 haloalkyl, cyclopropyl, and —NHR, wherein R is selected from the group consisting of C1-6 alkyl, C1-6 alkoxy, C1-6 alkanoyl, C1-6 hydroxyalkanoyl, C1-6 cyanoalkyl, C1-6 alkylamino, —(C1-6 alkylenyl)NH(CH3)—(C1-6 alkylenyl)N(CH3)2, and —(C1-3 alkylenyl)(3-7 membered-heterocyclyl);[0403]R3 and R4 are each independently selected from the group consisting of H, NH2, halo, C1-6 alkyl, C1-6 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C1-6 alkylthio, C1-6 haloalkylthio, C1-6 alkylamino, and cyclopropyl;[0404]R5 is selected from the group consisting of H, NH2, halo, C1-6 alkyl, C1-6 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C1-6 alkylthio, C1-6 haloalkylthio, C1-6 alkylamino, ...
embodiment 2
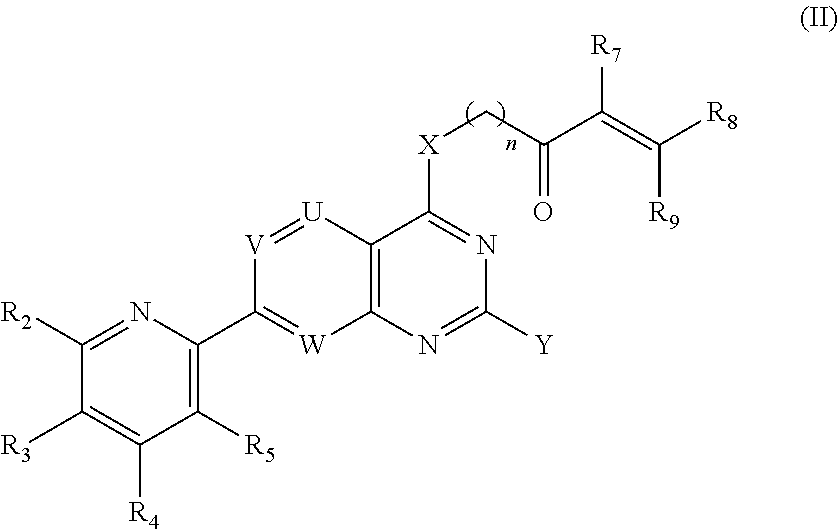
[0417]The compound of Embodiment 1 having a Formula (II):
[0418]or a pharmaceutically acceptable salt thereof;[0419]wherein,[0420]R2 is selected from the group consisting of H, OH, NH2, halo, C1-6 alkyl, C1-6 haloalkyl, cyclopropyl, and —NHR, wherein R is selected from the group consisting of C1-6 alkyl, C1-6 alkoxy, C1-6 alkanoyl, C1-6 hydroxyalkanoyl, C1-6 cyanoalkyl, C1-6 alkylamino, —(C1-6 alkylenyl)NH(CH3)—(C1-6 alkylenyl)N(CH3)2, and —(C1-3 alkylenyl)(3-7 membered-heterocyclyl);[0421]R3 and R4 are each independently selected from the group consisting of H, NH2, halo, C1-6 alkyl, C1-6 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C1-6 alkylthio, C1-6 haloalkylthio, C1-6 alkylamino, and cyclopropyl;[0422]R5 is selected from the group consisting of H, NH2, halo, C1-6 alkyl, C1-6 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C1-6 alkylthio, C1-6 haloalkylthio, C1-6 alkylamino, and C3-7 cycloalkyl,[0423]wherein at least one of R2, R3, R4, and R5 is other than H; or[0424]R2 and R3, R3 and R4, ...
embodiment 3
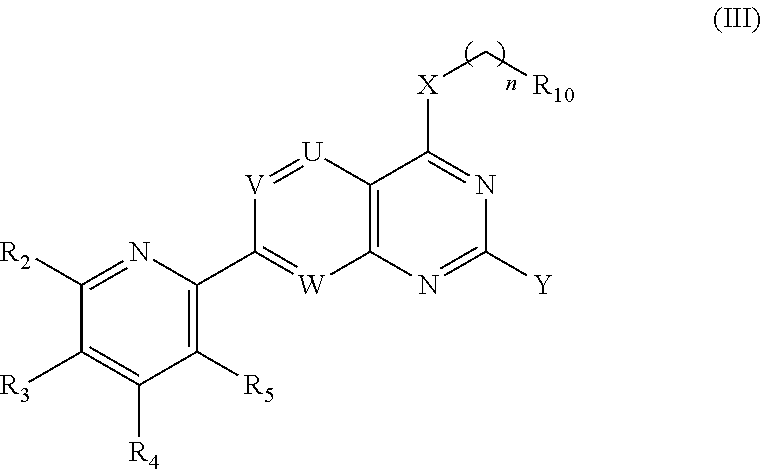
[0439]The compound of Embodiment 1 having a Formula (III):[0440]or a pharmaceutically acceptable salt thereof;
[0441]wherein,[0442]R2 is selected from the group consisting of H, OH, NH2, halo, C1-6 alkyl, C1-6 haloalkyl, cyclopropyl, and —NHR, wherein R is selected from the group consisting of C1-6 alkyl, C1-6 alkoxy, C1-6 alkanoyl, C1-6 hydroxyalkanoyl, C1-6 cyanoalkyl, C1-6 alkylamino, —(C1-6 alkylenyl)NH(CH3)—(C1-6 alkylenyl)N(CH3)2, and —(C1-3 alkylenyl)(3-7 membered-heterocyclyl);[0443]R3 and R4 are each independently selected from the group consisting of H, NH2, halo, C1-6 alkyl, C1-6 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C1-6 alkylthio, C1-6 haloalkylthio, C1-6 alkylamino, and cyclopropyl;[0444]R5 is selected from the group consisting of H, NH2, halo, C1-6 alkyl, C1-6 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C1-6 alkylthio, C1-6 haloalkylthio, C1-6 alkylamino, and C3-7 cycloalkyl,[0445]wherein at least one of R2, R3, R4, and R5 is other than H; or[0446]R2 and R3, R3 and R4,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com