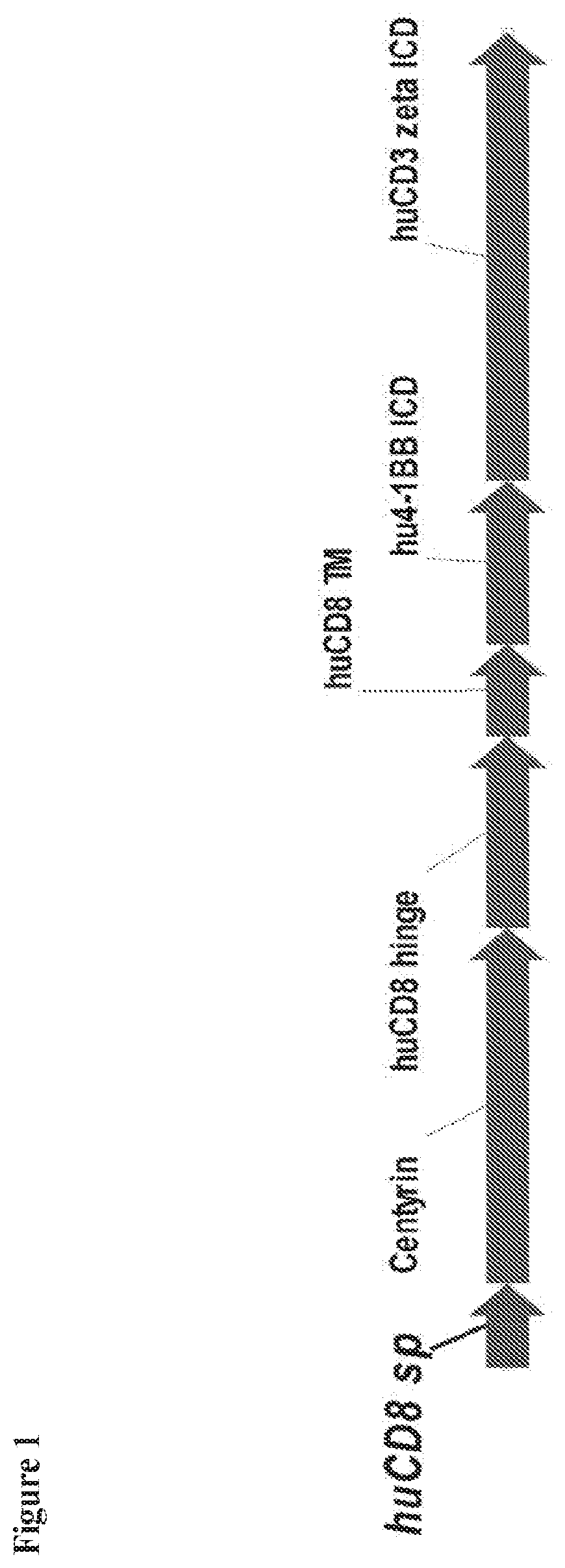
Chimeric antigen receptors comprising bcma-specific fibronectin type iii domains and uses thereof
a technology of chimeric antigen receptors and fibronectin, which is applied in the field of bcma-specific fibronectin type iii (fn3) domains, bcma-targeting chimeric antigen receptors, can solve the problem that scfvs are inherently more prone to biophysical challenges
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
f FN3 Domain Libraries Against Recombinant BCMA Proteins by CIS Display
[0217]Two constructs for BCMA recombinant protein target antigen were purchased from AB Biosiences. One was BCMA protein alone (catalog #P011Xp), and the other was BCMA fused to the Fc domain of mouse IgG2a (catalog #P001F).
[0218]FN3 Domain CIS-Display Library Selection
[0219]CIS-display was used to select BCMA-binding FN3 domains from the TCL18, TCL19, TCL21, TCL23, and TCL24 libraries.
[0220]TCL19, TCL21 and TCL23 Tencon libraries (SEQ ID NO: 46) are randomized at positions within the C and F strands and within the CD and FG loops, with the distribution of amino acids occurring at these positions as shown in Table 2. TCL19 and TCL21 were designed to include an equal distribution of 18 natural amino acids at every position (5.55% of each), excluding only cysteine and methionine. TCL23 was designed such that each randomized position approximates the amino acid distribution found in the CDR-H3 loops of functional an...
example 2
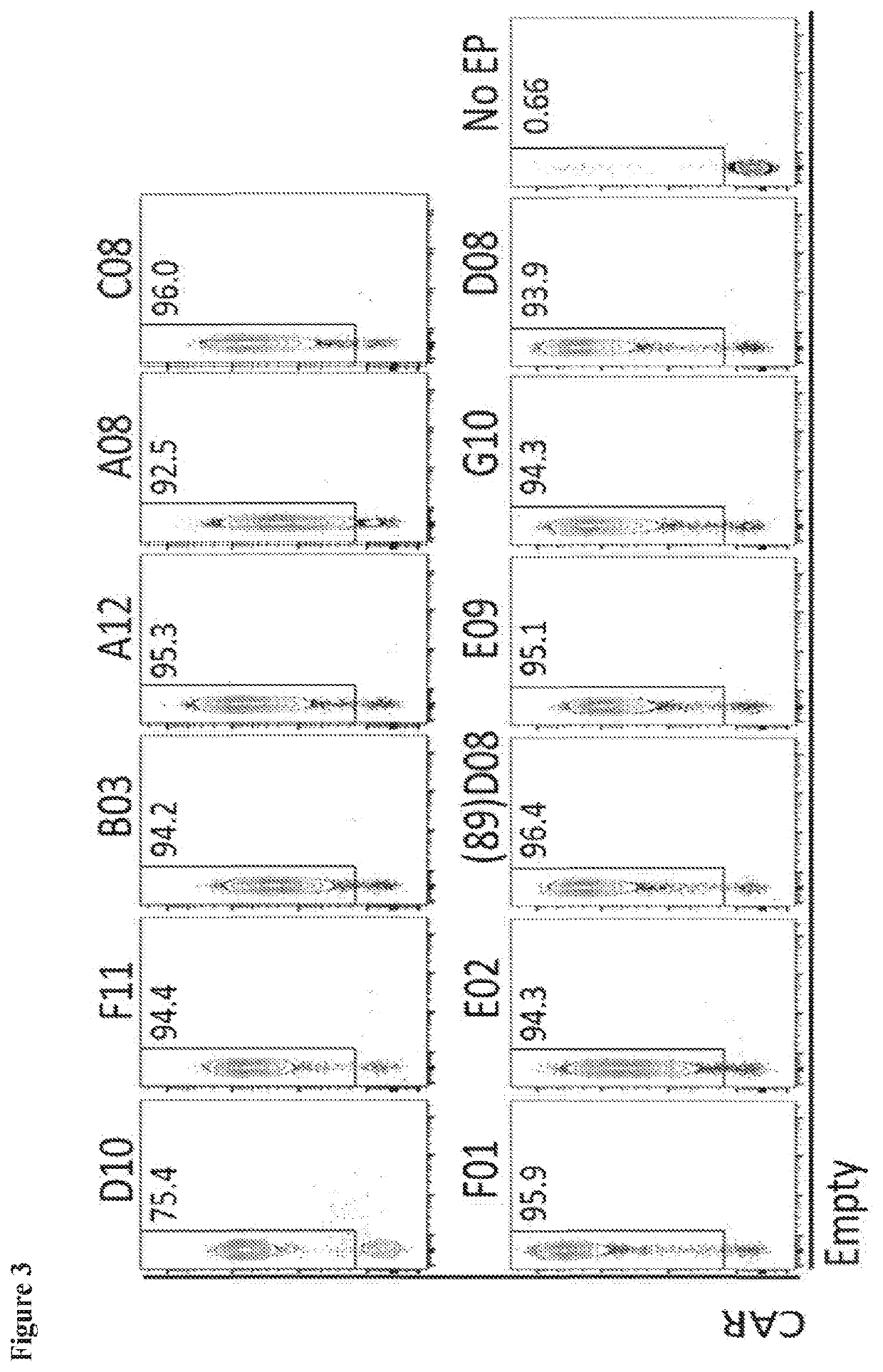
of BCMA-Specific FN3 Domains for Binding to HEK293F Cells Transiently Expressing Human BCMA
[0236]Cell counts and cell viability of hBCMA-expressing HEK293F and Mock HEK293F cells were measured, and 25×106 of each cell type were spun down at 175 g for 5 min. The media was aspirated and replaced with 25 mL FACS buffer. 100,000 cells were seeded per well in two V-bottom 96-well plates per cell type, and the plates were kept on ice throughout the experiment. The samples were set up as follows:
[0237]hBCMA cells; 2 uM treatment
[0238]hBCMA cells; 0.2 uM treatment
[0239]Mock cells; 2 uM treatment
[0240]Mock cells; 0.2 uM treatment
[0241]Positive and negative antibody controls were included. 12.5 uL of control antibodies were added to 238.5 uL FACS buffer to achieve 5 uL antibody (2.5 ug) per 100 uL treatment volume. Buffer controls were also included. The buffer control wells contained only 250 uL FACS buffer.
[0242]The plates were spun at 1200 rpm for 5 min at 4° C. to pellet the cells. Buffer...
example 3
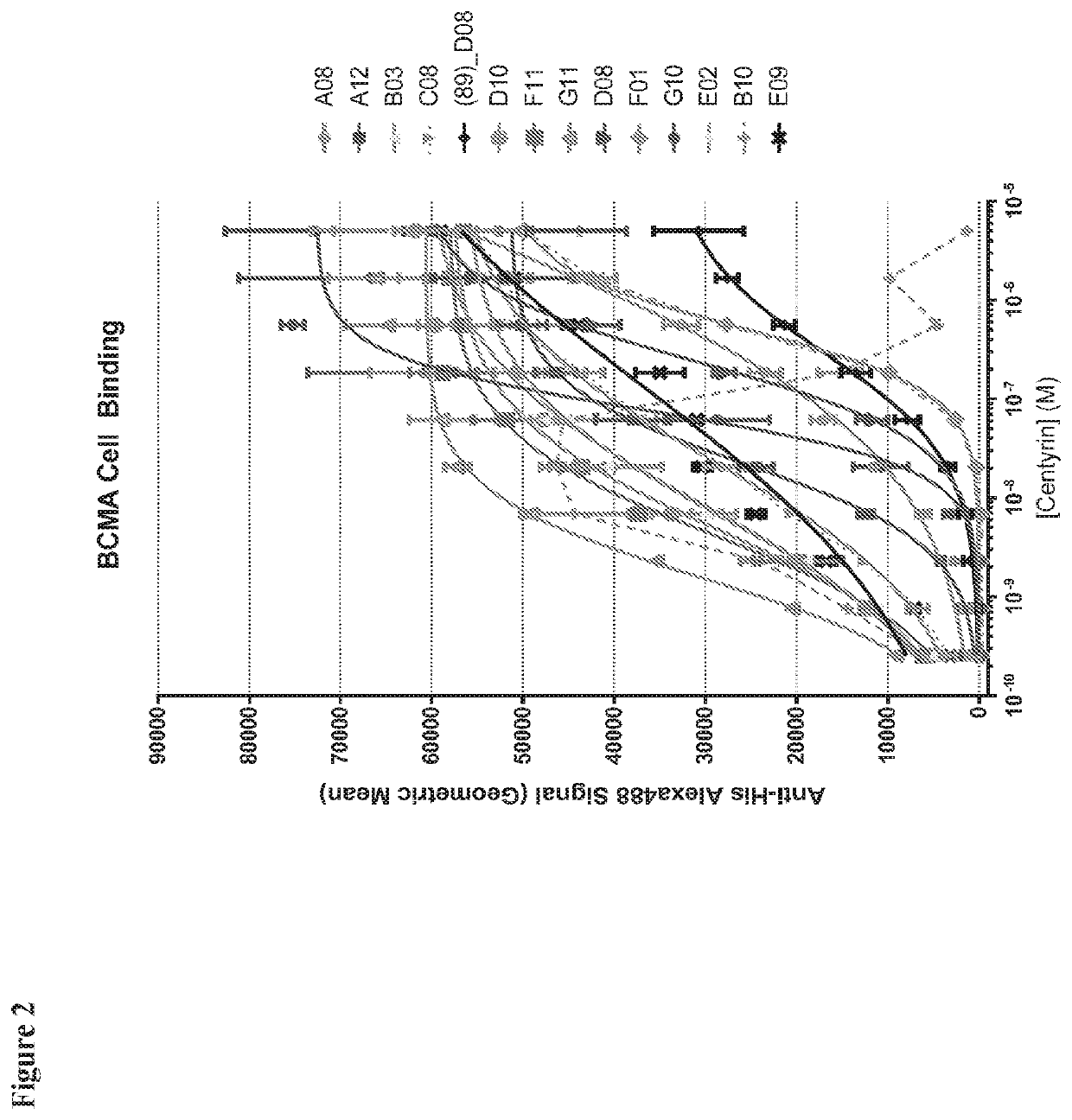
onse Curve (DRC) Assessment of Anti-BCMA FN3 Domains for Binding to HEK293F Cells Transiently Expressing Human BCMA
[0247]Ten 3-fold dilutions of FN3 domains were titrated in BD FACS buffer, with a starting concentration of 5 uM. Control samples were diluted in FACS buffer. Source plates, each with 7 FN3 domains in single-point dose titrations, were generated alongside a non-specific Tencon25 control for overlay onto HEK293F cells transiently expressing human BCMA. The assay controls included:
[0248]A Tencon25 dose-response curve series on each plate to measure non-specific binding;
[0249]Untreated cells stained with anti-His Alexa-488 to measure non-specific labeling;
[0250]Untreated cells with no anti-His antibody staining to measure absolute background;
[0251]A positive control of 1 uM of the FN3 domain referred to as ‘G10’ was used as a reference to control for plate-to-plate variability in signal intensity; and
[0252]Viability testing wells to troubleshoot apparent low viability.
[025...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com