Modified envelope glycoproteins for retroviridae viral vector pseudotyping and process for obtaining it
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
or Lentiviral Vectors Production and Titration
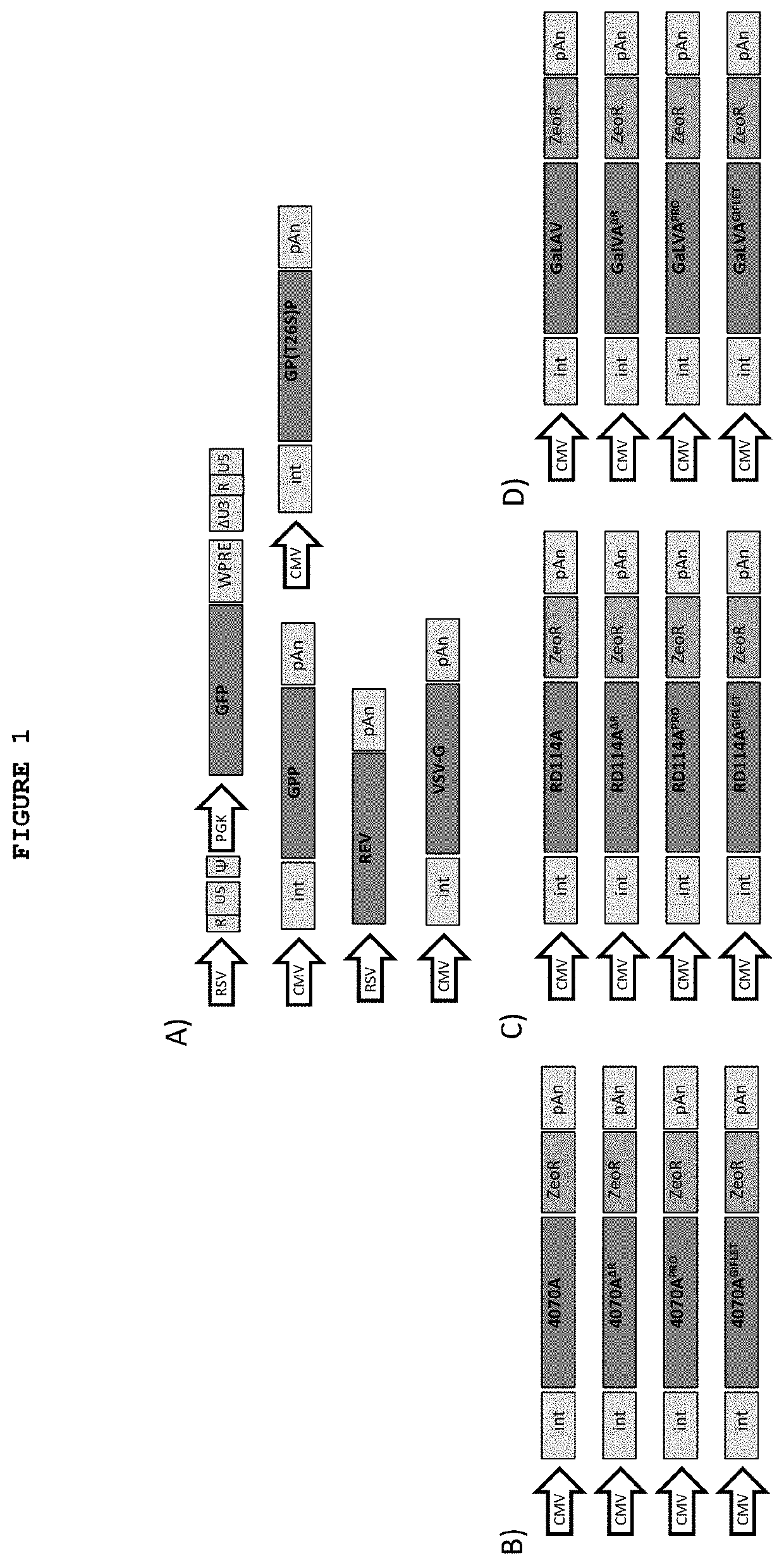
[0100]For transient production of lentiviral vectors, the third generation lentiviral packaging system and the transfection procedure as described in Tomás et al. (2013 and 2018) were used (Tomás, H. A., Rodrigues, A. F., Alves, P. M. Coroadinha, A. S. Lentiviral Gene Therapy Vectors: Challenges and Future Directions. Gene Therapy—Tools and Potential Applications (ed. Martin, F.). InTech (2013) pp. 287-317), (Tomás HA, Rodrigues A F, Carrondo M J T, Coroadinha A S. LentiPro26: novel stable cell lines for constitutive lentiviral vector production. Sci Rep. 8(1):5271. 2018).
[0101]The transfection procedure was conducted using PEI. HEK 293T cells were seeded at 5×104 cells / cm2 in 25 cm2 t-flask 24 h prior to transfection. A total of 4.65 μg of plasmid DNA per million cells was used for the transfection of one t-flask: 1 μg of pMDLg / pRRE or its variants (T26S and D25N) and 0.25 μg of pRSV-Rev (providing the packaging functions), 2.5 μg of pR...
example 2
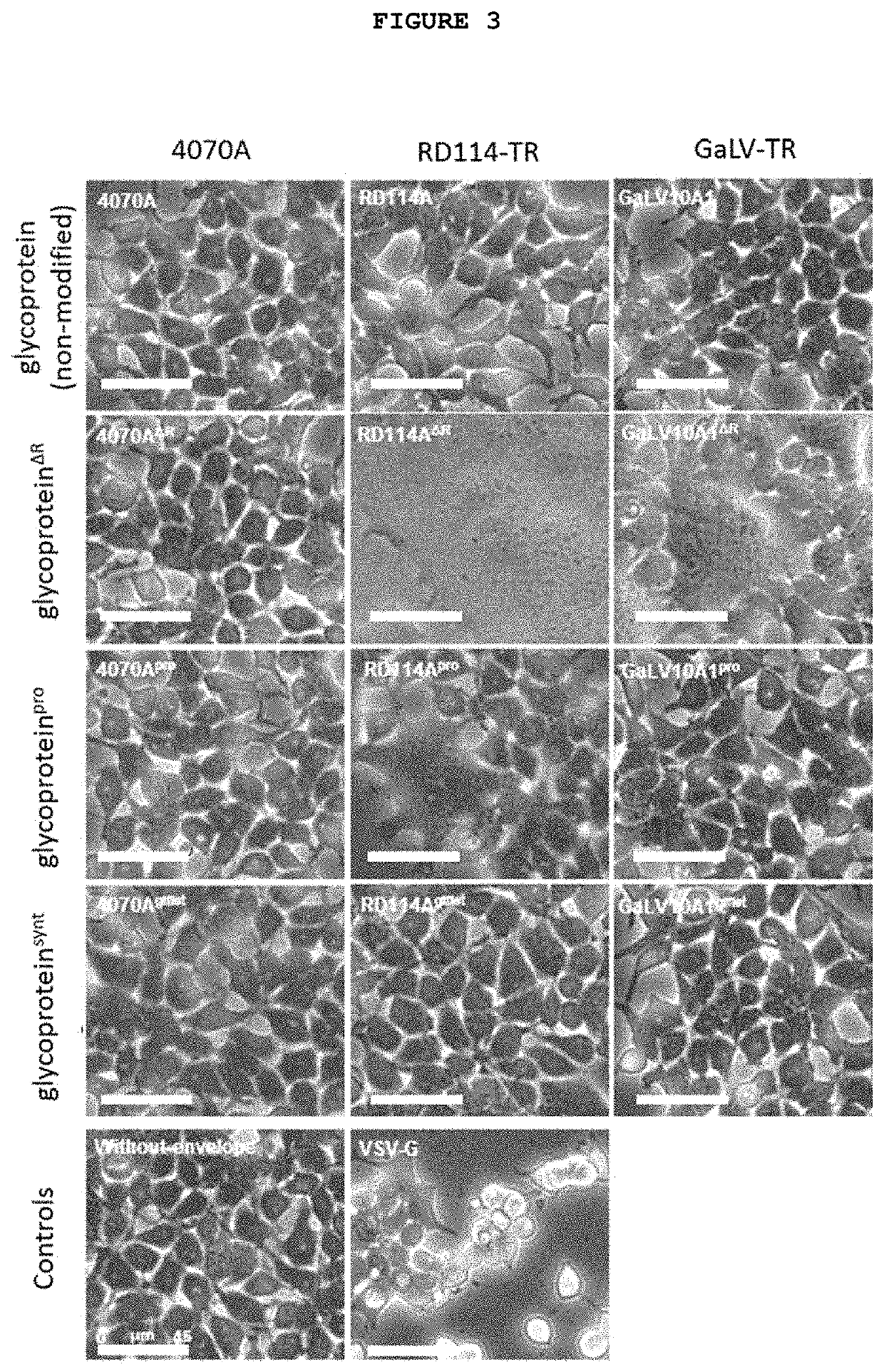
on of the Modified Envelope Glycoproteins for Viral Vector Pseudotyping
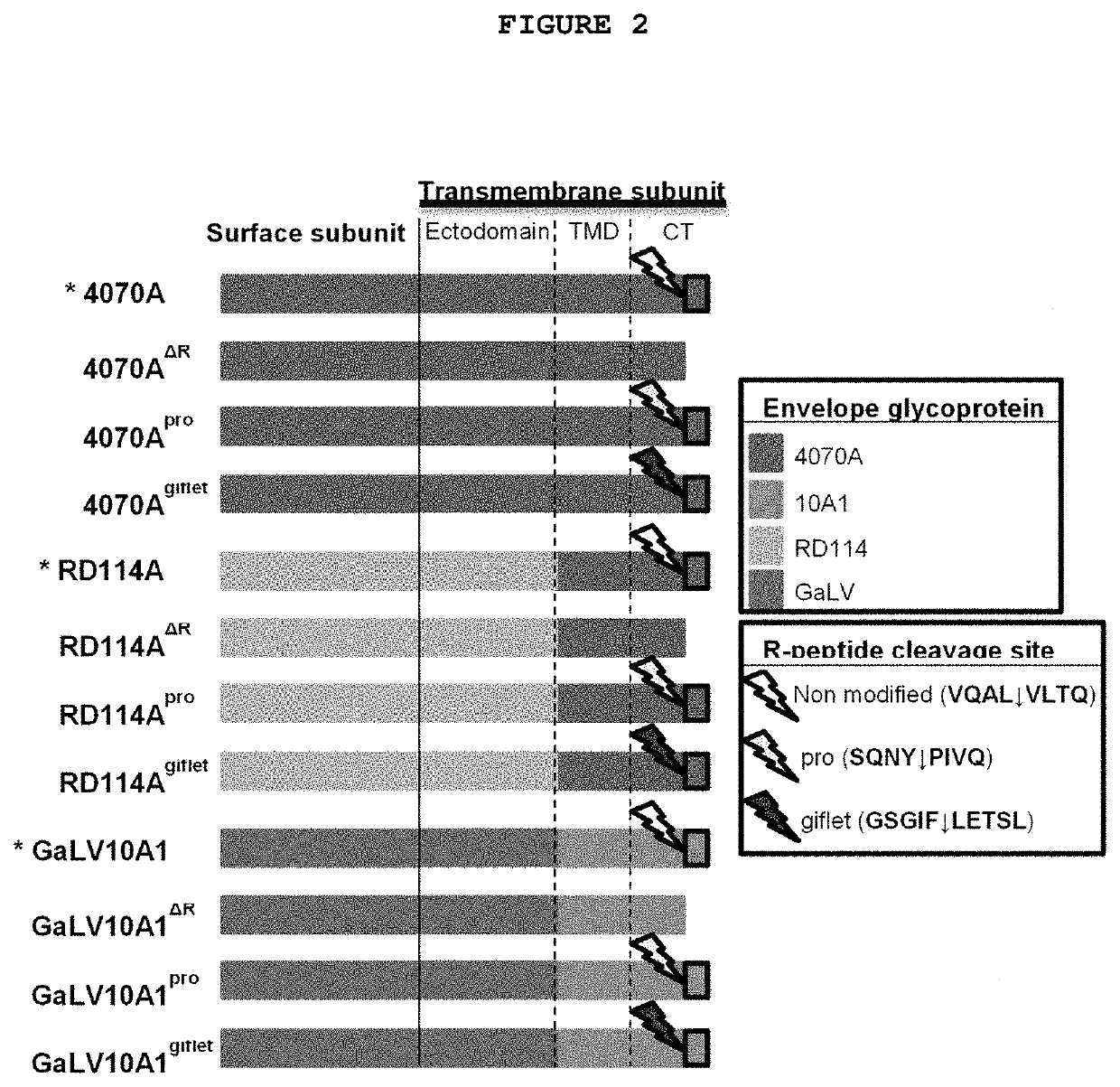
[0112]Gammaretrovirus envelope glycoproteins, unlike VSV-G, undergo proteolytic processing during virion assembly mediated by the retroviral protease. A short sequence—R-peptide—is cleaved from the cytoplasmic tail, as described by Tedbury, P. R. & Freed, E. O. (Tedbury, P. R. & Freed, E. O. The cytoplasmic tail of retroviral envelope glycoproteins. Prog. Mol. Biol. Transl. Sci. 129, 253-84. 2015). This cleavage is required for virus entry, since it activates the fusogenic activity of the envelope glycoprotein.
[0113]The R-peptide cleavage site in the original envelope glycoproteins is specifically recognized by the retroviral protease. The efficiency of cleavage is dependent on both the sequence of cleavage and the protease used (i.e. its virus family origin and introduced mutations). Enhanced cleavage is expected when (i) homologous cleavage sequences, in relation to the viral protease, are used and (ii) highly ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap