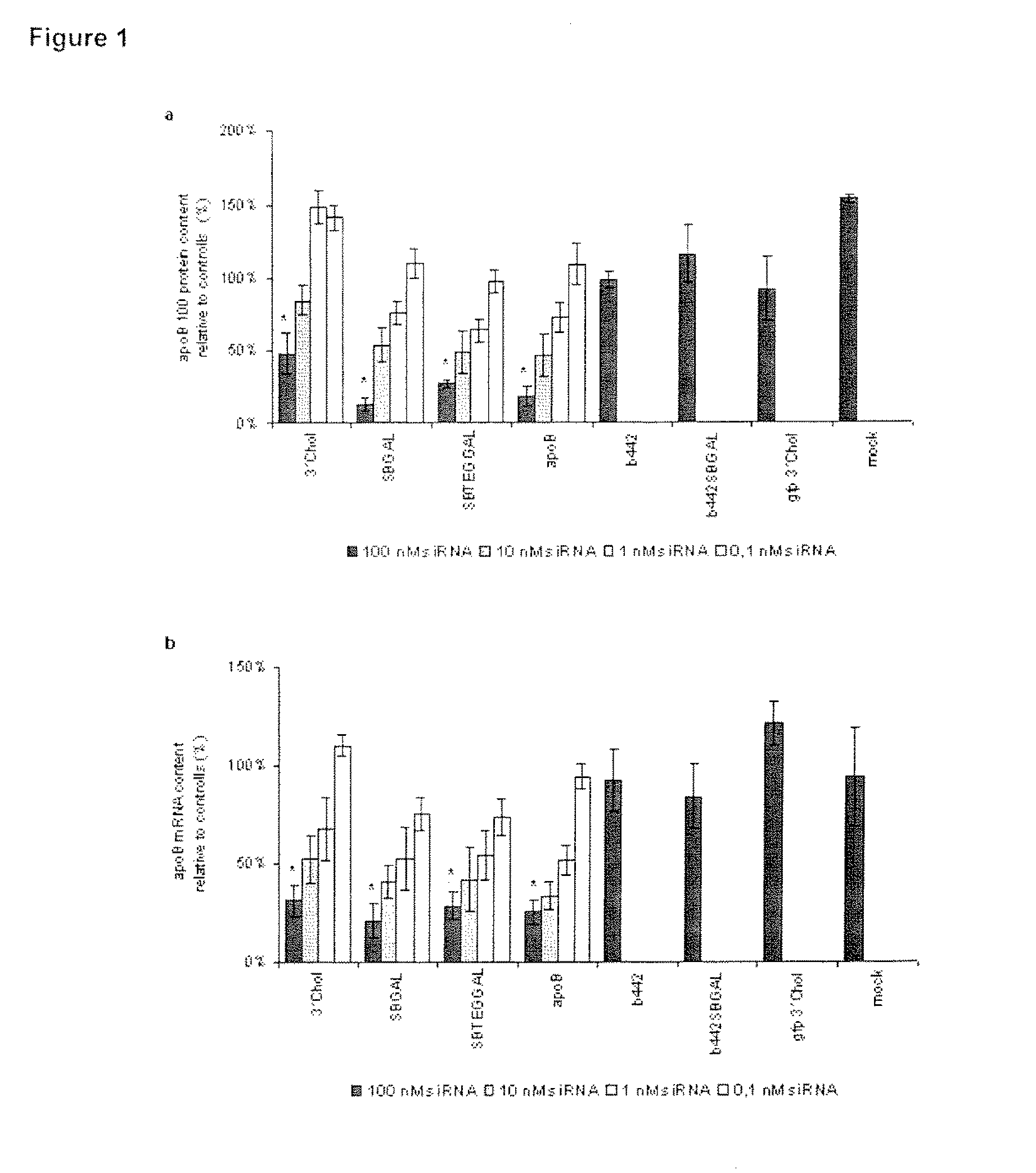
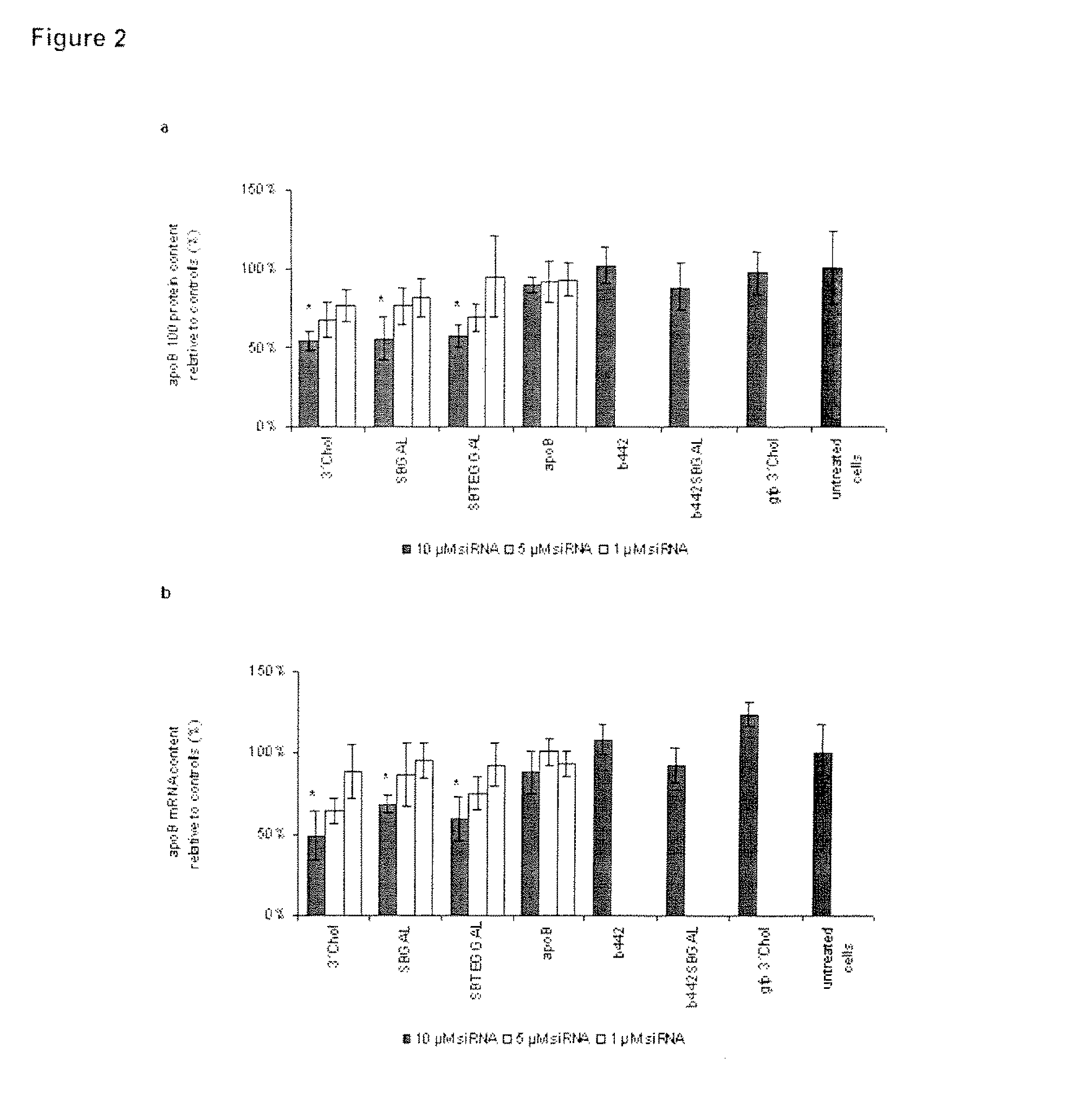
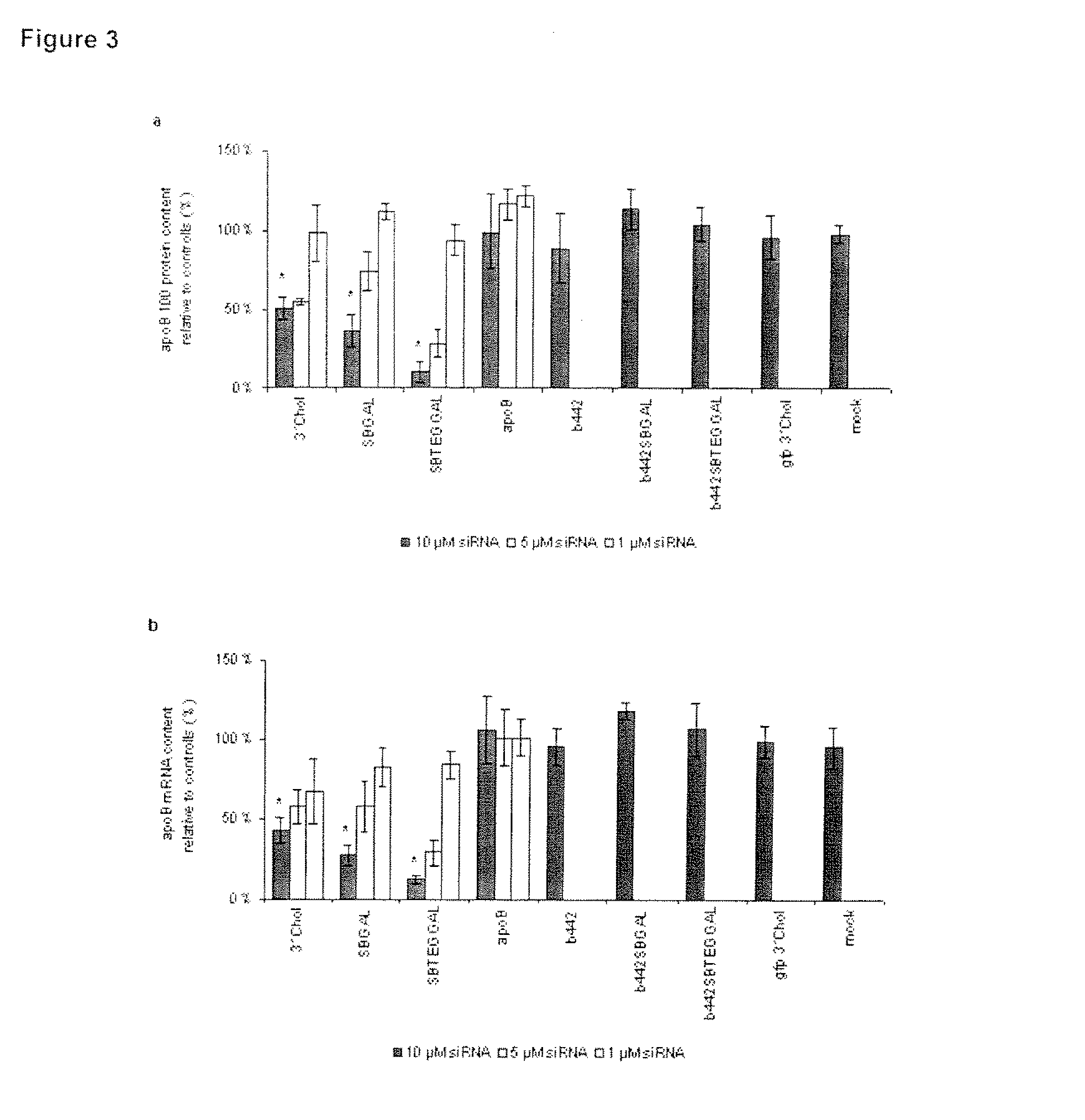
Glycoconjugates of RNA interference agents
a technology of rna interference and glycoconjugates, which is applied in the direction of sugar derivatives, biochemistry apparatus and processes, organic chemistry, etc., can solve the problems of insufficient safety and/or efficiency of nucleic acid delivery systems, the inability of nucleic acid molecule to disrupt the natural functions of cells as little as possible, and the inability to meet the needs of cell function, etc., to inhibit the expression of a target gene, the effect of inhibiting th
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
1. Materials
[0266]Where the source of a reagent is not specifically given herein, such reagent may be obtained from any supplier of reagents for molecular biology at a quality / purity standard for application in molecular biology.
Synthesis of 1-O-{4-[(2-cyanoethoxy)-N,N-diisopropylamino-phosphanyloxy]-butyl}-6-O-(4-methoxytriphenylmethyl)-2,3,4-tri—O-acetyl-B-D-galactopyranoside (9)
[0267]The synthesis of compound 9 is illustrated in FIG. 6. 20 g (51.24 mmol) of β-D-galactosepentaacetate 1 was dissolved in 150 ml THF and 6.7 ml (61.49 mmol) of benzylamine was added with a dropping funnel. The reaction was stirred for 18 h at room temperature to give 2,3,4,6-tetra-O-acetyl-β-D-galactopyranose 2.
[0268]Product 2 was dissolved in 50 ml (240 mmol) trichloacetonitrile and cooled down to −20° C. Within 15 min 3.56 ml (23.96 mmol) of 1,8-Diazabicyclo[5.4.0]-undec-7-ene were added using a dropping funnel. After 1.5 h synthesis of 2,3,4,6-tetra-O-acetyl-D-galactopyranosyl-trichloroacetimidate 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com