Method for preparation of N-acetyl cysteine amide
a technology of n-acetyl cysteine and amide, which is applied in the field of preparation of n-acetyl cysteine amide, can solve the problems of limited therapeutic usefulness of gsh precursors, and achieve the effects of high chemical yield and high chemical and enantiomeric purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Experiments
[0028]The following procedures may be employed for the preparation of the compound of the present invention. The starting materials and reagents used in preparing these compounds are either available from commercial suppliers such as the Aldrich Chemical Company (Milwaukee, Wis.), Bachem (Torrance, Calif.), Sigma (St. Louis, Mo.), or are prepared by methods well known to a person of ordinary skill in the art, following procedures described in such references as Fieser and Fieser's Reagents for Organic Synthesis, vols. 1-17, John Wiley and Sons, New York, N.Y., 1991; Rodd's Chemistry of Carbon Compounds, vols. 1-5 and supps., Elsevier Science Publishers, 1989; Organic Reactions, vols. 1-40, John Wiley and Sons, New York, N.Y., 1991; March J.: Advanced Organic Chemistry, 4th ed., John Wiley and Sons, New York, N.Y.; and Larock: Comprehensive Organic Transformations, VCH Publishers, New York, 1989.
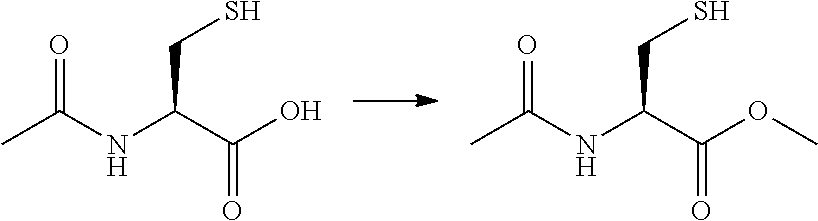
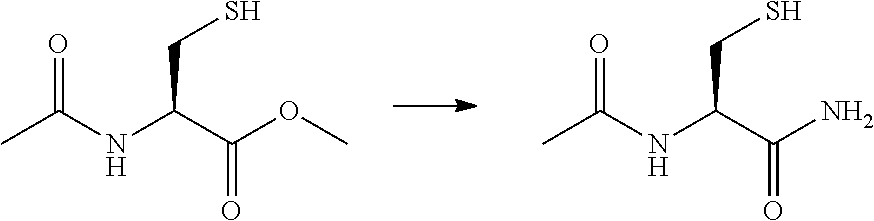
[0029]Preparation of N-Acetyl Cysteine Amide (NACA)
[0030]
[0031]N-Acetyl Cystei...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| organic | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com