Acetamide derivative having defined particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
We first briefly describe the drawings.
I. Drawings
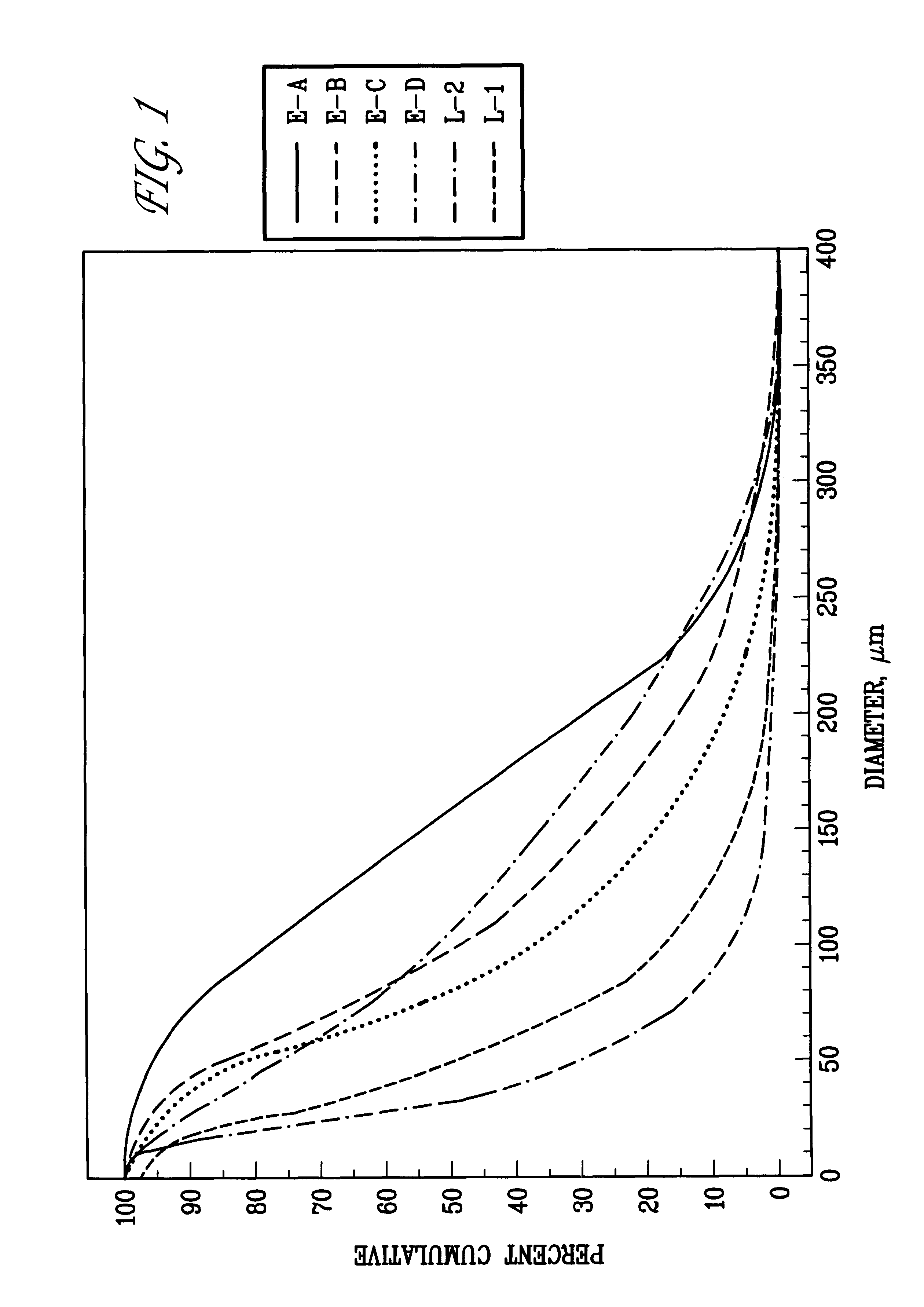
FIG. 1 is a graph depicting particle size distributions for six lots of modafinil: Lots L-1, L-2, E-A, E-B, E-C and E-D.
FIG. 2 is a scanning electron micrograph of a sample of modafinil Lot E-D at 50.times. magnification.
FIG. 3 is a scanning electron micrograph of a sample of modafinil Lot E-D at 100.times. magnification.
FIG. 4 is a scanning electron micrograph of a sample of modafinil Lot L-1 at 50.times. magnification.
FIG. 5 is a scanning electron micrograph of a sample of modafinil Lot L-1 at 100.times. magnification.
FIG. 6 is a graph depicting the dissolution rate of modafinil particles from Lot E-D (median particle size 94.05 .mu.m) and Lot L-1 (median particle size 50.18 .mu.m).
FIG. 7 is a graph depicting the dissolution rate of modafinil particles from Lot E-B (median particle size 89.10 .mu.m), Lot E-D (median particle size 94.05 .mu.m) and Lot L-1 (median particle size 50.18 .mu.m).
FIG. 8 is a graph depicting mean plasma con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size distribution | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com