Production of 1,1,1,2-tetrafluoroethykane and pentafuoethane simultaneously
A technology for pentafluoroethane and tetrafluoroethane, applied in the field of preparing fluorine-containing alkanes, can solve the problems such as no disclosure of R123 manufacturing method, inability to simultaneously prepare R134a and R125, and inability to simultaneously prepare R134A and R125, etc. Equipment cost and energy consumption cost, the effect of reducing the types of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
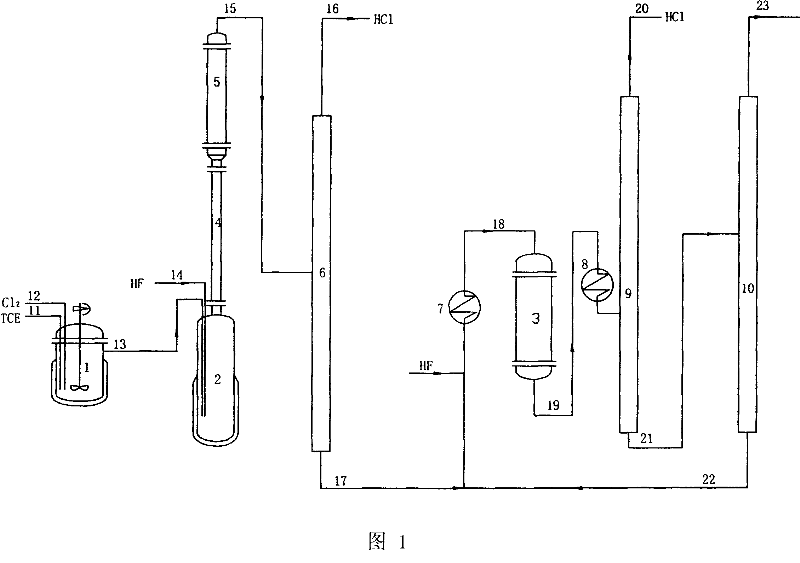
[0023] The first step reaction and the second step reaction are respectively reacted in two reactors, and the production process of simultaneously manufacturing R134a and R125 is:
[0024] 1. Preparation of Catalyst No. 1. Add 732.5g (500ml) TCE, 50g reduced iron powder in a 1 liter four-neck flask, install booster stirrer, reflux condenser, pass Cl 2 tube and temperature measuring tube, turn on the stirrer, cool the reflux condenser with water, heat the four-necked flask with a water bath, control the temperature in the flask to 70°C, and then pass Cl 2 Reaction, reflux condenser top unreacted Cl 2 Guide to absorb in 10% sodium hydroxide solution, pass through Cl 2 The speed is about 0.5 liters / minute, until all the reduced iron powder reacts, that is, an anhydrous iron trichloride halogenated hydrocarbon solution is obtained, wherein the solution contains FeCl 3 15.30%, this solution is No. 1 catalyst.
[0025] 2. Preparation of Catalyst No. 2. Add 32.6g of No. 1 cataly...
Embodiment 2
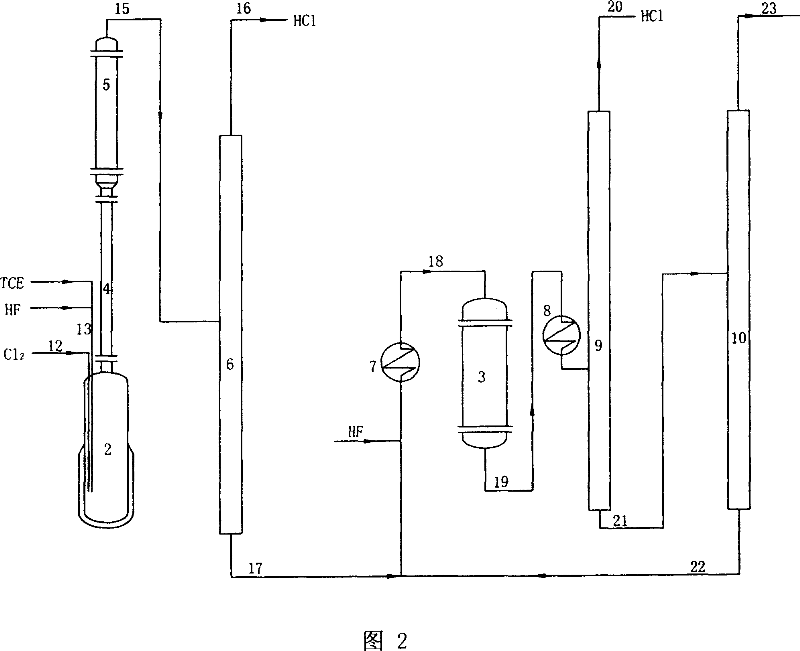
[0029] The first step reaction and the second step reaction are completed in the same reactor, and the production process for simultaneously manufacturing R134a and R125 is:
[0030] 1. Preparation of Catalyst No. 1. The preparation of No. 1 catalyst is the same as in Example 1.
[0031] 2. Preparation of Catalyst No. 2. The preparation of No. 2 catalyst is the same as in Example 1.
[0032] 3. Preparation of Catalyst No. 3. The preparation of No. 3 catalyst is the same as in Example 1.
[0033] 4. Arrange equipment according to the technological process of accompanying drawing 2, wherein, the first step reaction and the second step reaction all react in reactor 2, add No. 1 catalyst 0.3 liters in reactor 2, No. 2 catalyst 1.4 liters; TCE Feeding rate is 700g / h, Cl 2 The molar ratio of TCE to TCE is 1.5; the molar ratio of HF to TCE in reactor 2 is 3.3; the molar ratio of HF to organic matter in reactor 3 is 9. Others are identical with embodiment 4. After the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com