Preparation process of key intermediate 5-cyanphthalide of antidepressant drug citalopram
A technology for citalopram and antidepressants, which is applied in the field of pharmaceutical intermediate preparation, can solve the problems of complex post-processing, harsh conditions, long cycle time, etc., and achieves the effects of short reaction process flow, simple material feeding and post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
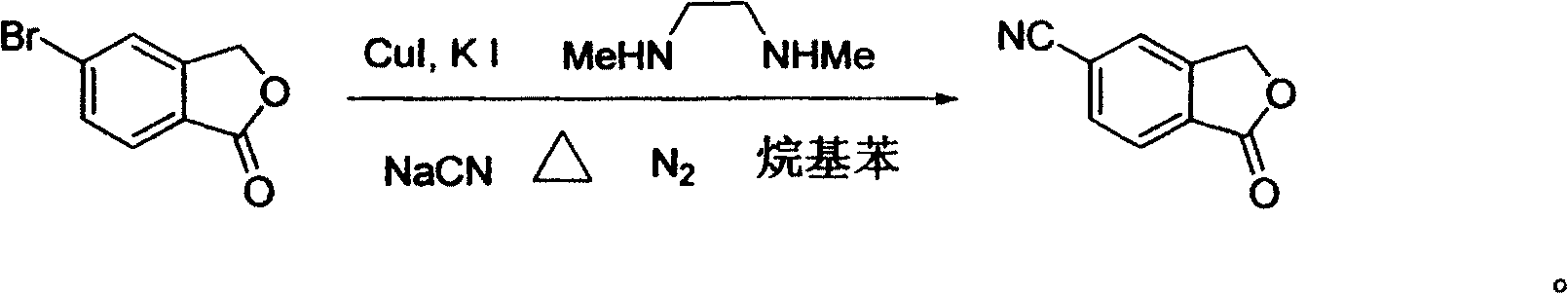
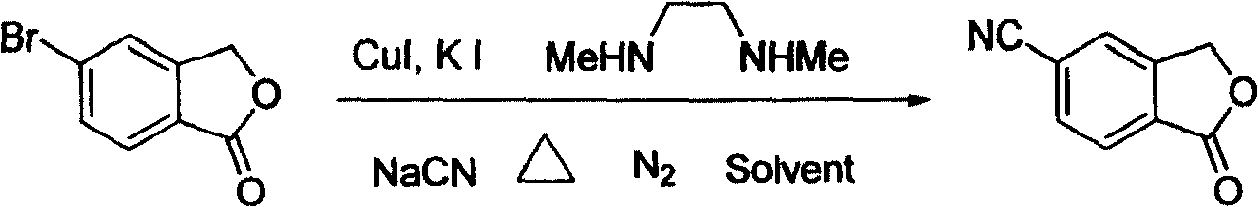
[0017] In a 100 ml three-necked flask, 50 ml of toluene, 6.4 g (30 mmol) of 5-bromophthalide, 1.18 g (36 mmol, 1.2 equivalents) of sodium cyanide, 0.573 g (3 mmol) of sodium cyanide were added successively under nitrogen protection. , 0.1 equivalent) cuprous iodide, 1 gram of potassium iodide (6 mmol, 0.2 equivalent), 2.64 grams of N, N'-dimethylethylenediamine (30 mmol, 1.0 equivalent), under nitrogen protection at 110 ° C Stir the reaction for 30 hours, end the reaction, recover the solvent toluene under reduced pressure, then add 50 ml of water to the residue, stir at room temperature for 1 hour, filter, wash with water, and finally the filtrate is separated by recrystallization with ethanol to obtain white needle-shaped crystals of 5-cyanobenzene Phthale, the yield is 90%, the purity is 98% (HPLC), and the melting point is 200-202°C. 1 HNMR (CDCl 3 , ppm): 5.51(2H, s), 7.55(1H, s), 7.55(1H, s), 8.05(1H, d, J=2.5Hz).
Embodiment 2
[0019] In a 100 ml three-necked flask, 60 ml of ethylbenzene, 6.4 g (30 mmol) of 5-bromophthalide, 1.6 g (39 mmol, 1.3 equivalents) of sodium cyanide, 0.573 g (3 mg) of sodium cyanide were added successively under nitrogen protection. mol, 0.1 equivalent) cuprous iodide, 1 gram of potassium iodide (6 mmol, 0.2 equivalent), 2.64 grams of N, N'-dimethylethylenediamine (30 mmol, 1.0 equivalent), under nitrogen protection at 130 Stir the reaction at ℃ for 24 hours, end the reaction, recover the solvent ethylbenzene under reduced pressure, then add 50 ml of water to the residue, stir at room temperature for 1 hour, filter, wash with water, and finally the filtrate is separated by recrystallization from ethanol to obtain white needle-shaped crystals of 5-cyanide phenylphthalide, the yield is 85%, the purity is 99%, and the melting point is 201-202°C.
Embodiment 3
[0021] In a 100 ml three-necked flask, 50 ml of toluene, 6.4 g (30 mmol) of 5-bromophthalide, 1.18 g (36 mmol, 1.2 equivalents) of sodium cyanide, 0.7 g (4.5 mmol) of sodium cyanide were added successively under nitrogen protection. , 0.15 equivalents) cuprous iodide, 1.5 grams of potassium iodide (9 mmoles, 0.3 equivalents), 2.64 grams of N, N'-dimethylethylenediamine (30 mmoles, 1.0 equivalents), under nitrogen protection at 100 ° C Stir the reaction for 48 hours, end the reaction, recover the solvent toluene under reduced pressure, then add 50 ml of water to the residue, stir at room temperature for 1 hour, filter, wash with water, and finally the filtrate is separated by recrystallization with ethanol to obtain white needle-shaped crystals of 5-cyanobenzene Phthale, the yield is 91%, the purity is 98%, and the melting point is 200-202°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com