Pharmaceutical composition containing prostaglandin
A technology of prostaglandin and composition, which is applied in the field of pharmaceutical composition of oil-in-water emulsion, can solve the problem of content decline and achieve the effect of inhibiting decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
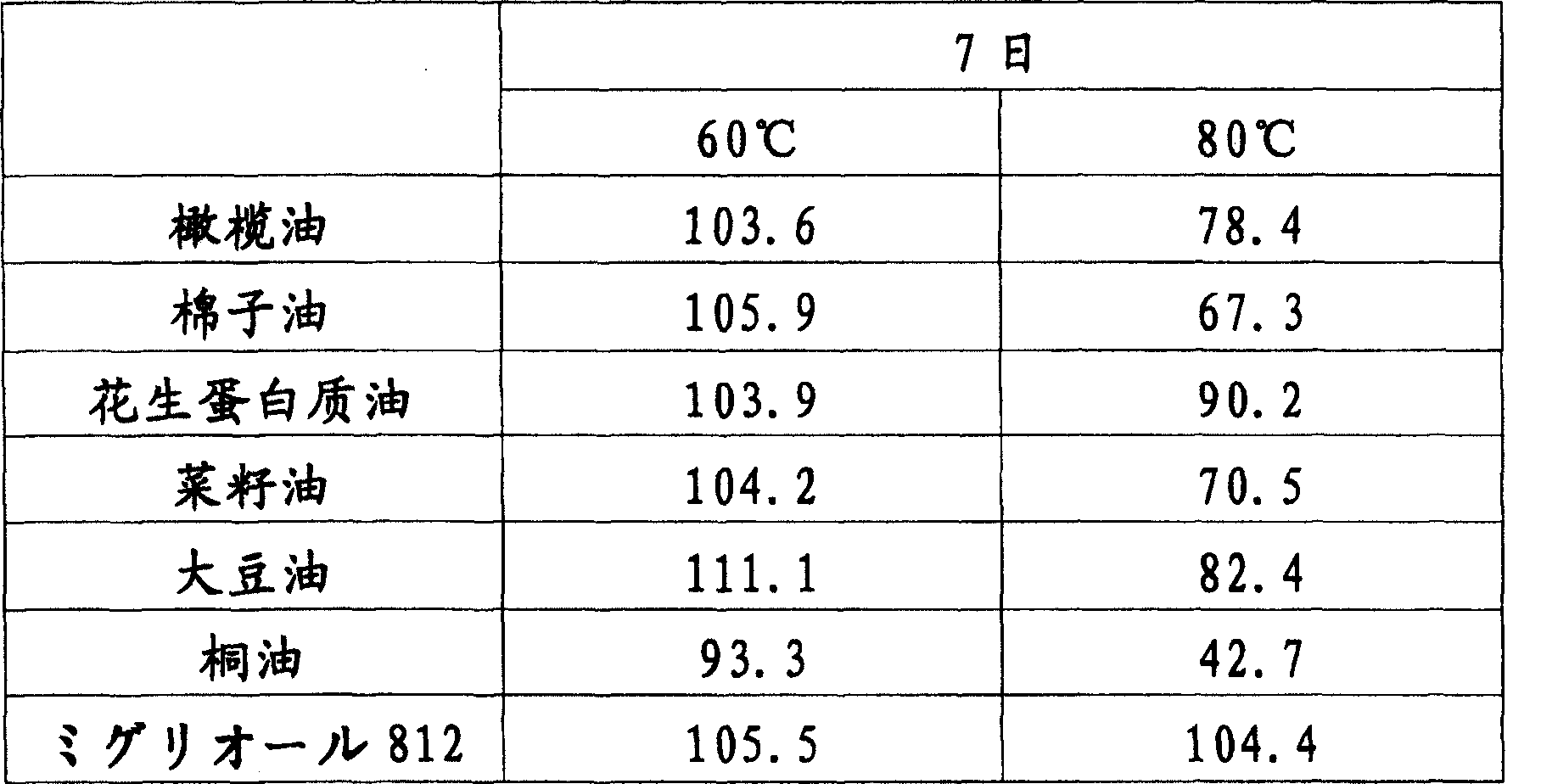
Image
Examples
Embodiment 1
[0076] [Formulation Example 1] Eye Drops
[0077] Latanoprost 0.01g
[0078] Concentrated Glycerin 2.4g
[0079] Polyvinyl alcohol 2g
[0080] Sodium acetate 0.1g
[0081] Sodium edetate 0.01g
[0082] Chlorhexidine Gluconate Solution (20W / V%) 0.025mL
[0083] ミダリオ—ル812 1g
[0084] Hydrochloric acid amount
[0085] Purified water The total volume becomes 100mL
[0086] pH 7.0
[0087] According to the above prescription, disperse concentrated glycerin and polyvinyl alcohol in an aqueous solution of sodium acetate, heat it, and dissolve it while vigorously stirring with a homomixer, then add migelio-lu dissolved in latanoprost to make it emulsified. Cool to room temperature, add edetate sodium, chlorhexidine gluconate solution, add 1N hydrochloric acid to adjust the pH, mix well, filter and sterilize to prepare eye drops.
Embodiment 2
[0088] [Formulation Example 2] Eye Drops
[0089] Latanoprost 0.005g
[0090] Concentrated Glycerin 2.4g
[0091] Boric acid 1.6g
[0092] Sodium edetate 0.01g
[0093] Sorbic acid 0.2g
[0094] Hydroxypropyl methylcellulose 0.9g
[0095] ミゲリオ—ル812 0.6g
[0096] Hydrochloric acid amount
[0097] Purified water The total volume becomes 100mL
[0098] pH 7.0
[0099] According to the above prescription, slowly disperse concentrated glycerin and hydroxypropyl methylcellulose in the boric acid aqueous solution, heat, and dissolve while vigorously stirring with a homomixer, then add the latanoprost-dissolved ミゲリオール, make of emulsification. Cool to room temperature, add sodium edetate, sorbic acid, and hydrochloric acid to adjust the pH, mix well, filter and sterilize to prepare eye drops.
Embodiment 3
[0100] [Formulation Example 3] Eye Drops
[0101] Latanoprost 0.005g
[0102] Concentrated Glycerin 2.4g
[0103] Sodium acetate 0.1g
[0104] Sodium edetate 0.01g
[0105] Sorbic acid 0.2g
[0106] Xanthan gum 0.9g
[0107] Peanut protein oil 0.6g
[0108] Hydrochloric acid amount
[0109] Purified water The total volume becomes 100mL
[0110] pH 6.0
[0111] According to the above prescription, slowly disperse concentrated glycerin and xanthan gum in an aqueous solution of sodium acetate, heat, dissolve while vigorously stirring with a homomixer, and then add Migliol in which latanoprost is dissolved to emulsify it. Cool to room temperature, add sodium edetate, sorbic acid, and 1N hydrochloric acid to adjust the pH, mix well, filter and sterilize to prepare eye drops.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com