Peptides modulating caspase activation
A purpose and active site technology, applied in the field of drugs and the death mechanism of human and animal cells, can solve the problem of not finding the special effect of CRGDVLDCL
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] peptide synthesis
[0038] Peptides were synthesized using a Synthesizer Model 430A; Applied Biosystems, Foster City, CA, USA, according to common methods for peptide synthesis. Cyclic peptides are mainly formed spontaneously from linear peptides in aqueous solution exposed to atmospheric oxygen and are purified by HPLC, using usual procedures for peptides.
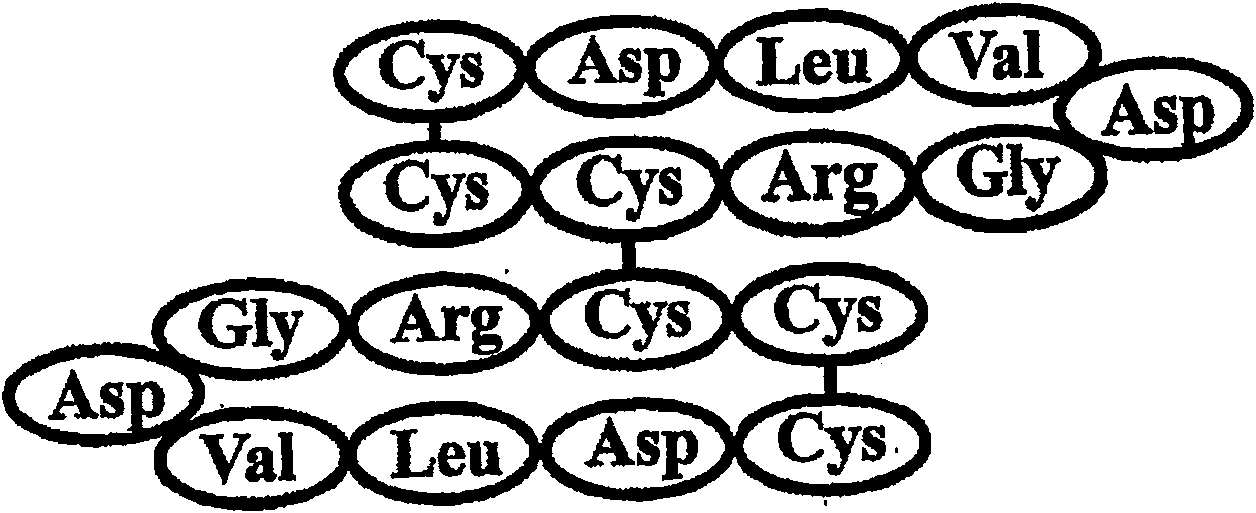
[0039] 1. Corresponding to the cyclic polymeric 9-peptide *Cys-*Cys-Arg-Gly-Asp-Val-Leu-Asp-Cys* corresponding to the 251-259 amino acid sequence in human AFP, *CCRGDVLDC* (the asterisk indicates a potential two sulfur bond), referred to herein as apocyclin-A. The second cysteine, Cys252, is capable of forming an S-S bond between two adjacent cyclic monomeric peptides. The molecular weight of the monomeric cyclic peptide is 983 Da. The apocyclin-A peptide consists of two or more (less than 5) cyclic 9-peptide structures corresponding to the sequence *Cys-*Cys-Arg-Gly-Asp-Val-Leu-Asp-Cys*.
[0040]
[0041] 2...
Embodiment 2
[0058] Example 2 Identification of the RGD-containing caspase-3-specific active site responsible for apoptotic signaling in human AFP
[0059] To determine the functionally important amino acids in the AFP molecule, we determined the amino acid homology of the sequences of human AFP and caspase-3 to caspase-1 and constructed peptide analogs of putative active sites. Align the following sequences:
[0060] casp-2 QNKPKMFFI QUR ETDRGV
[0061] :::::
[0062] casp-1 KDKPKVIII QUR SPGVVW
[0063] :::::
[0064] casp-3 TGKPKLFII QACRGTE LDC GI
[0065] :::. :::
[0066] HuAFP VLDVAHVHEH CCRGDVLDC LQDGEKIMSYICSQQDTLSNKITECCKLTTLERGQCIIHAE 299
[0067] : :. :: :: ::.: : : . ::: .::..::. ::: ::. :: :
[0068] HSA VTDLTKVHTE CCHGDLLEC ADDRADLAKYICENQDSISSKLKECCEKPLLEKSHCIAEVE 294
[0069] It can be seen from the comparison that AFP and caspase-1 have certain homology with the enzyme active sites of caspase-3. The...
Embodiment 3
[0070] Example 3 Cyclic peptide from AFP apocyclin-A eliminates AFP-induced apoptosis in U937 cells
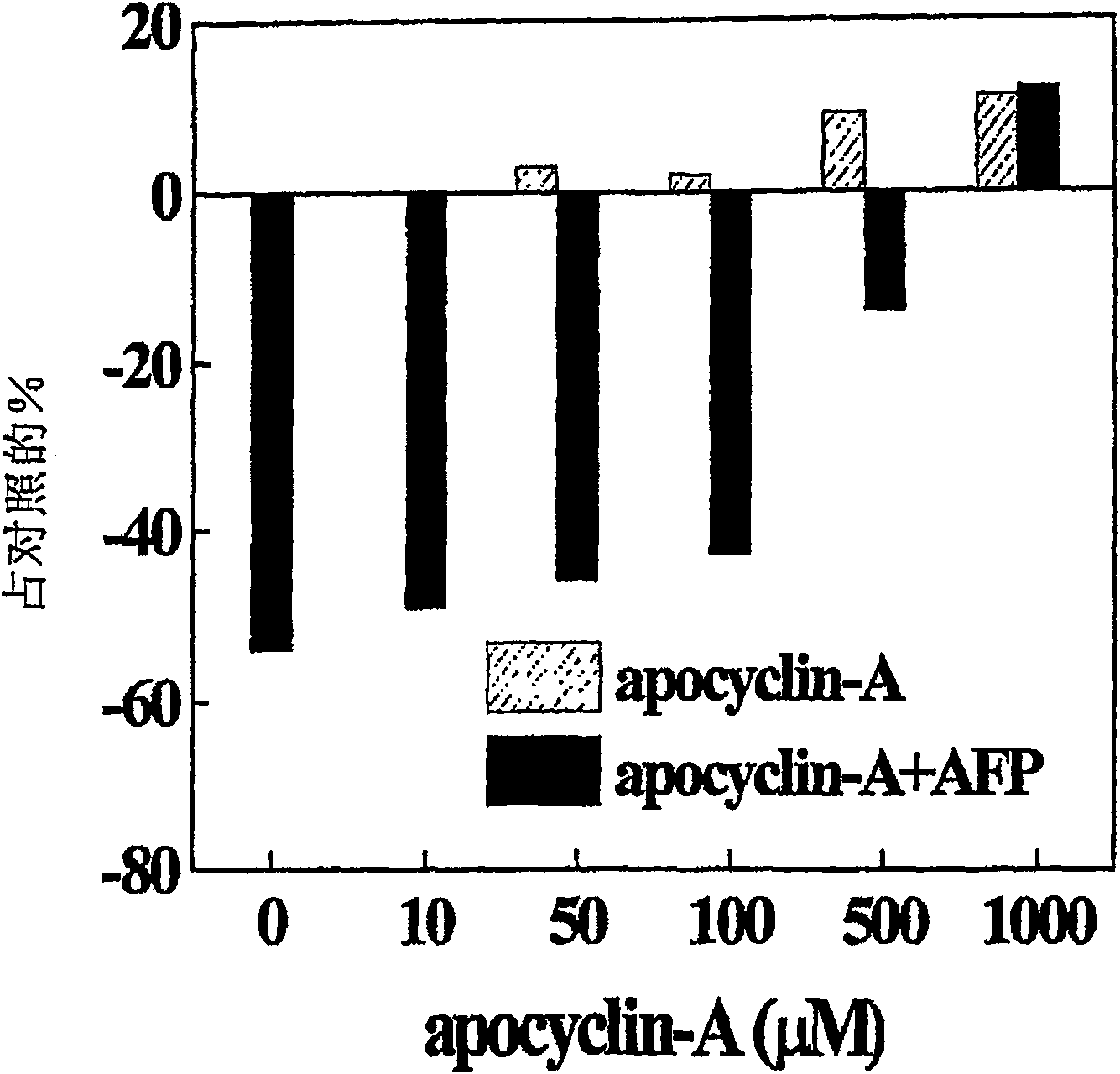
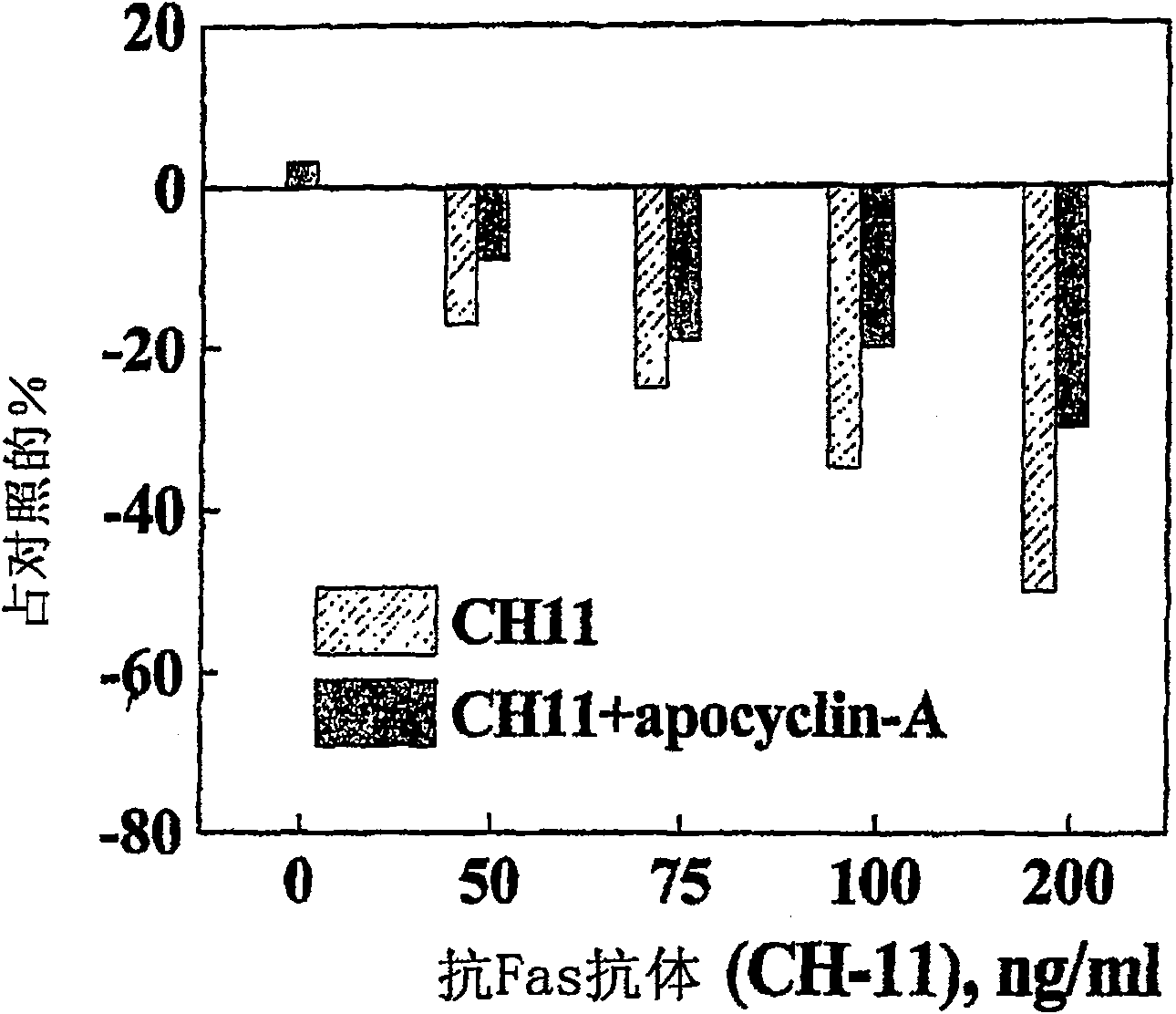
[0071] Previously published data demonstrated that AFP of different origins (embryonic or derived from the culture medium of the hepatoma cell line HepG2) could induce growth inhibition and dose-dependent programmed cell death in various tumor cell lines, characterized by a typical Apoptotic features such as marked growth inhibition, cytotoxicity and DNA fragmentation (Dudich et al., (1998) Tumor Biol. 19:30-40). To determine the possible localization of the active site on the AFP molecule responsible for apoptotic signaling, we tested the ability of each AFP-derived peptide to block this effect. figure 2 It shows that AFP-derived peptide apocyclin-A completely eliminates AFP-induced apoptosis in U-937 cells. In addition, 15-minute pretreatment of U-937 cells with apocyclin-A (1 mM) induced a 15% stimulation of proliferation compared to medium control. This means that apocy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com