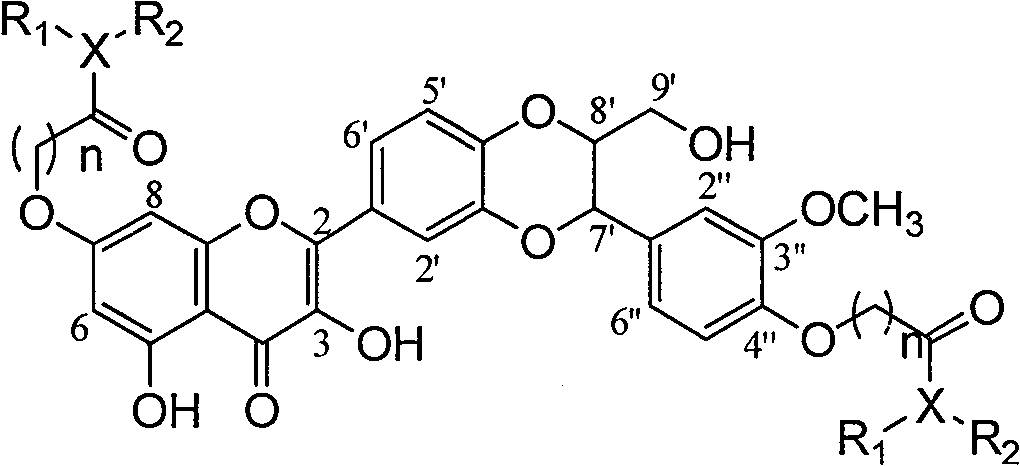
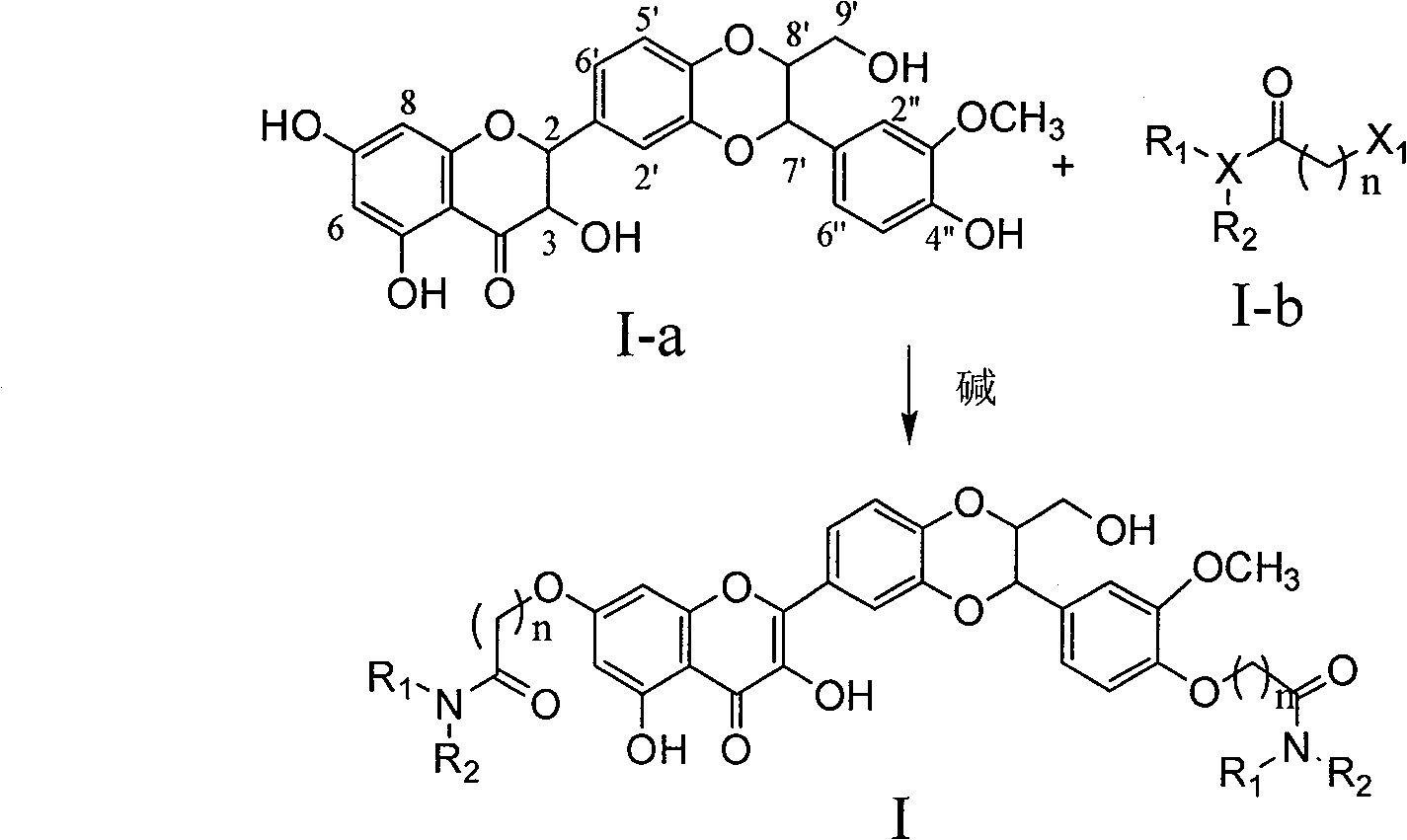
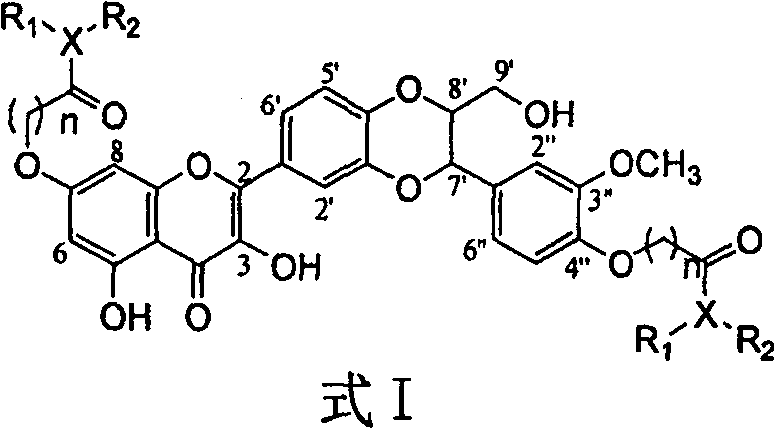
Dehydrosilibinin diester derivatives, preparation method and use thereof
A technology of dehydrogenated water and derivatives, applied in the field of organic chemistry and medicinal chemistry, can solve the problems of limited drug market, insufficient water solubility and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Compound I-1 (2-[2,3-dihydro-3-(4-N,N-diethylcarbamoylmethoxy-3-methylhydrogenphenyl)-2-hydroxymethyl-1 ,4-benzodioxane-6-]-7-(4-N,N-diethylcarbamoylmethoxy-3-methoxyphenyl)-3,5,-dihydroxy- 4H-1-benzopyran-4-one) preparation:
[0030]
[0031]2.2 grams of compound I-a (silibinin) were dissolved in 15 milliliters of N,N-dimethylformamide, 1 gram of potassium carbonate and 0.15 grams of potassium iodide were added, and 0.67 grams of N,N-diethylchloroacetamide was added under stirring , stirred overnight at room temperature, the reactant was poured into 30 ml of ice water, and the yellow bag precipitate was precipitated, extracted with ethyl acetate, washed with saturated brine after combining the extracts, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a yellow solid. 1.2 g of yellow powder was obtained by column chromatography with a yield of 60%.
[0032] Rf (chloroform: methanol = 50: 1) = 0.1; 1 H NMR (400MHz, deuterated di...
Embodiment 8
[0044] Embodiment 8: Compound I-8 ([2-[2,3-dihydro-3-(4-ethoxyformylmethoxy-3-methoxyphenyl)-2-hydroxymethyl-1,4-benzo Dioxane-6-]-2,3-dihydro-7-(4-ethoxyformylmethoxy-3-methoxyphenyl)-3,5,-dihydroxy-4H-1- The preparation of benzopyran-4-one]):
[0045]
[0046] According to the same method as in Example 1, the raw material ethyl chloroacetate was used instead of N, N-diethyl chloroacetamide to obtain compound I-8: Rf (chloroform: ethyl acetate: formic acid=25:1:0.25)=0.10 ; 1 H NMR (400MHz, deuterated dimethylsulfoxide): 1.23(t, J=7.2Hz, 3H, CH3), 1.31(t, J=7.2Hz, 3H, CH3), 3.58(m, 1H, 9'a ), 3.86(m, 1H, H-9'b), 4.13(m, 1H, H-8'), 4.20(q, J=7.2Hz, 2H, CH2CH3), 4.28(q, J=7.2Hz, 2H, CH2CH3), 4.28 (m, 1H, H-8'), 4.67 (s, 2H, H-9), 4.82 (s, 2H, OCH2CO), 5.99 (d, J=8.0Hz, 1H, OCH2CO) , 6.35(s, 1H, H-6), 6.41(s, 1H, H-8), 6.96-8.01(m, 6H, Ar-H), 12.50(s, 1H, 5-OH); ESI-MS 653[M+1] + .
[0047]The compounds of formula I have various important biological activities. The p...
Embodiment 2
[0059] Pharmacological embodiment 2: Activity Test of Compound I-2 in Vitro Scavenging Diphenylpicrylhydrazyl (1,1-diphenyl-2-picrylhydrazyl, DPPH)
[0060] The methanol solution of DPPH has a strong absorption value at 517nm. When it is reduced by antioxidants, the absorption value decreases. The lower the absorbance, the stronger the antioxidant effect. In a 250 μL reaction system, 25 μL of compound I-2 with a concentration of 40 μg / mL, 40 μL of methanol solution of DPPH (0.4 mg / mL) and 185 μL of methanol solution were contained, and the absorbance was measured at 517 nm after reacting in a water bath at 37 °C for 30 minutes. The methanol solution of DPPH and the same concentration of quercetin were used as negative and positive controls, respectively. The test results are shown in Table 3.
[0061] Table three
[0062]
[0063]
[0064] The test results show that compound I-2 has a certain DPPH free radical scavenging effect, but at the same concentration, its sca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com