Preventive and/or therapeutic agent for diabetes
A technology for diabetes and glycogen, which is applied in pharmaceutical formulations, metabolic diseases, drug combinations, etc., can solve problems such as hyperactive energy metabolism and unclear mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] experiment method
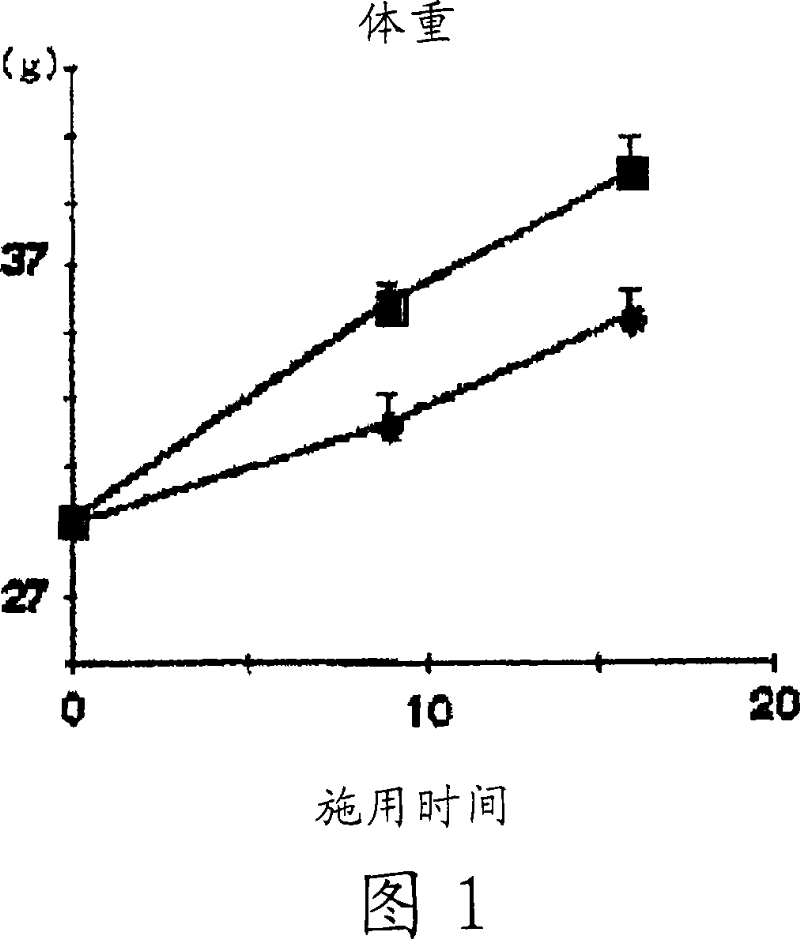
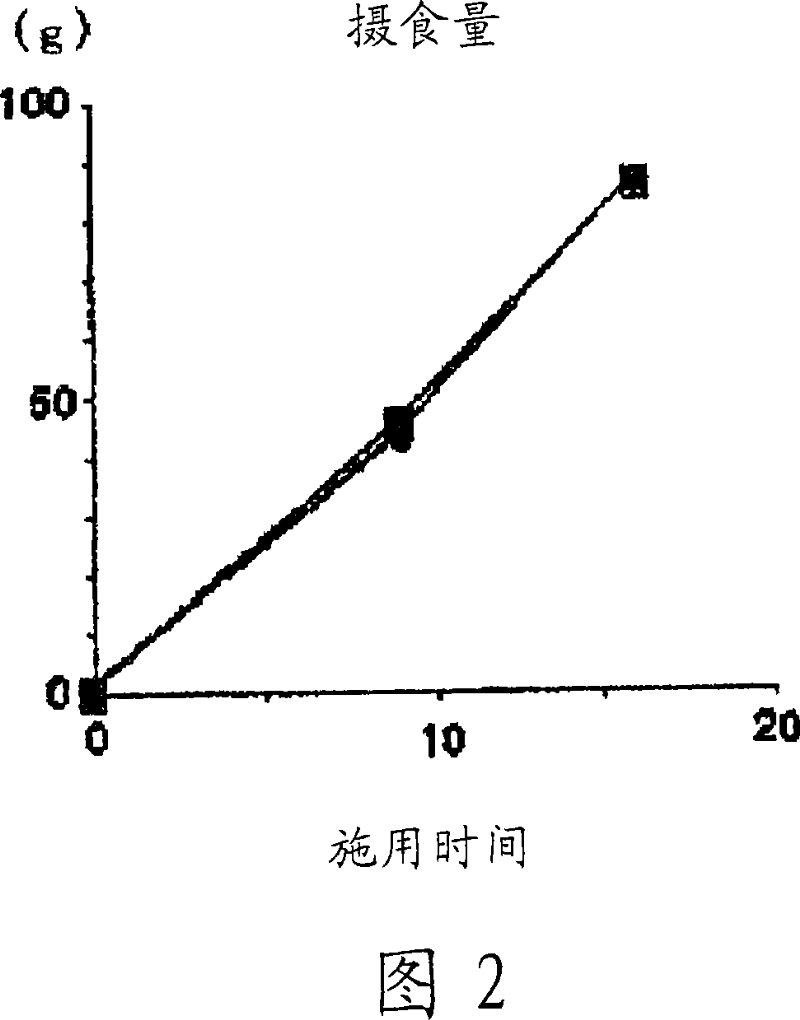
[0088] KKAy mice (male, Japanese CLEA, 5 weeks old, N=4-5) were used. The control group was fed normal food, and the cholemide group was fed normal food containing 2% cholemide (UAR company (Villemoison sur Orge, France)).
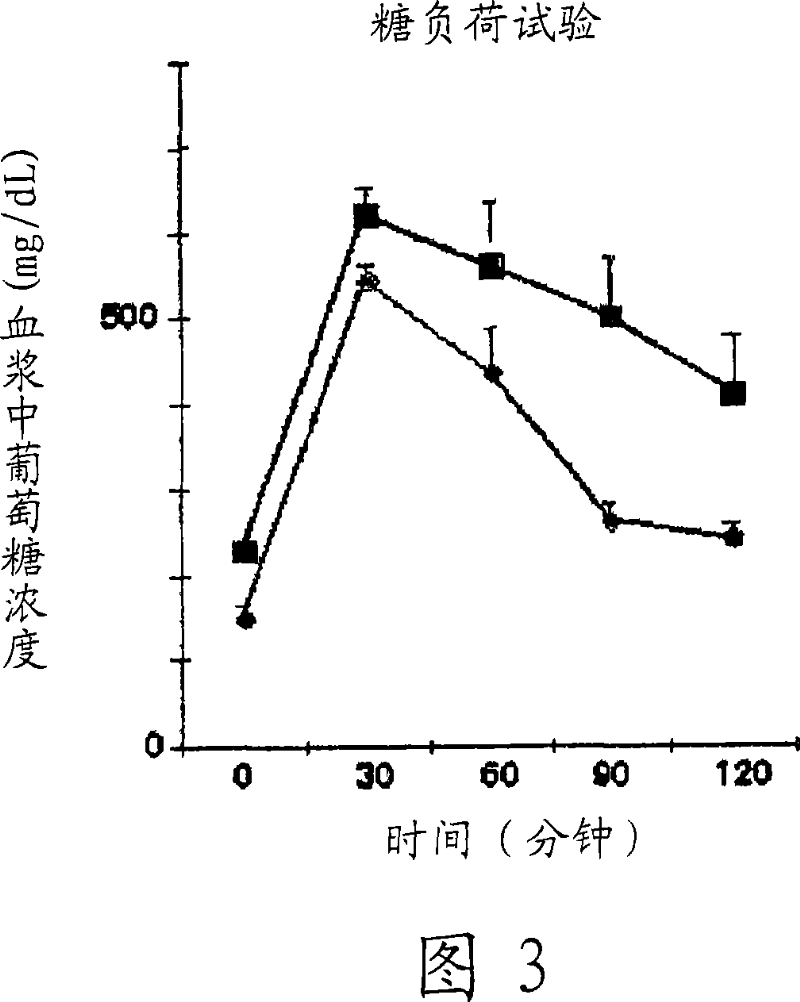
[0089] After 12 days, an oral glucose load test was carried out according to the usual method. Blood was collected before the glucose load, and then a glucose solution was orally administered at a ratio of 2 g / kg body weight, and blood glucose values were measured 30, 60, 90, and 120 minutes later.
[0090] After 1 week of the experiment, the livers of the mice were taken out, and the gene expression levels (correlated mRNA levels) of the bile acid synthase cyp7α, the inhibitory nuclear receptor SHP, the glycogenesis enzymes PEPCK and G6Pase were analyzed by real-time quantitative PCR. Determination.
[0091]The primer sequences used for the real-time quantitative PCR reaction are respectively, the sequences of the sequence ...
Embodiment 2
[0106] experiment method
[0107] KKAy mice (male, Japanese CLEA, 5 weeks old, N=6) were used. GW-4064 (J. Med. Chem. 2000, Vol. 43 (16), pp. 2971-2974), which is an FXR agonist, was orally administered at a dose of 10 mg / kg for four days. On day 5, the mice were dissected and the livers were removed. The gene expressions of cyp7α, SHP and PEPCK in the liver were determined by quantitative PCR. In addition, the primer sequences used in the real-time quantitative PCR reaction are the same as those in Example 1.
[0108] result
[0109] (1) Gene expression of cyp7α and SHP in the liver
[0110] The results are shown in FIG. 6 . Compared with the control group, the gene expression level of cyp7α decreased in the GW-4064 group (FIG. 6-1), while the gene expression level of SHP increased (FIG. 6-2). This result indicates that GW-4064, which is an FXR agonist, negatively regulates the synthesis of bile acids in the liver due to the increase of FXR activity.
[0111] (2) Gene ...
Embodiment 3
[0115] experiment method
[0116] C57BL6 mice (male, Charles River Laboratories (l'Arbresle, France), 5 weeks old, N=4-5) were used. Guggulipid is a general drug used as a drug for treating hyperlipidemia. In the present invention, the drug sold by Syntrax Innovations is used.
[0117] The control group (HFD-Control) was fed a high-fat diet (35.9% fat, UAR company (Villemoison sur Orge, France)), the Mukul group (HFD-Mukul) was fed a diet containing 2.5% Mukul Kool-Myrrh high-fat diet, colemide group (HDF-Colemid) was fed a high-fat diet containing 2% colemide.
[0118] Insulin loading test was performed according to the usual method at the 8th week of administration. Blood was collected before insulin load, and insulin was injected intraperitoneally, and blood glucose levels were measured 30, 60, and 90 minutes later.
[0119] At the 9th week of administration, an oral glucose load test was performed according to the usual method. Blood was collected before the glucose lo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com