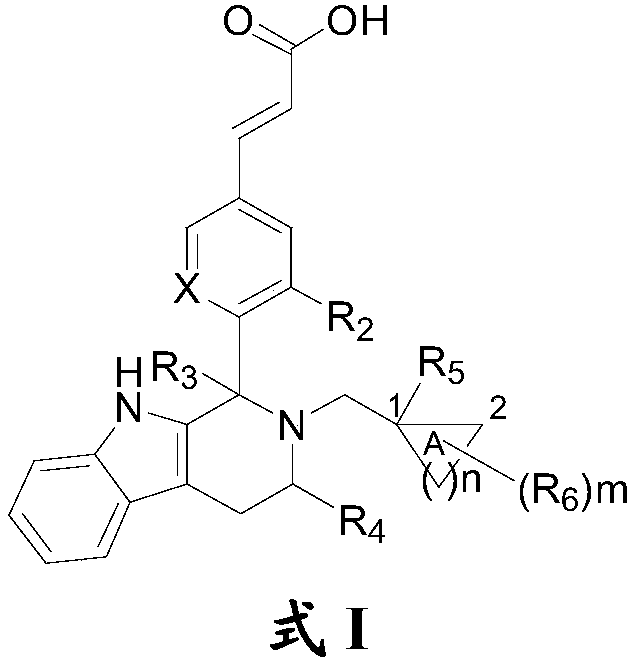
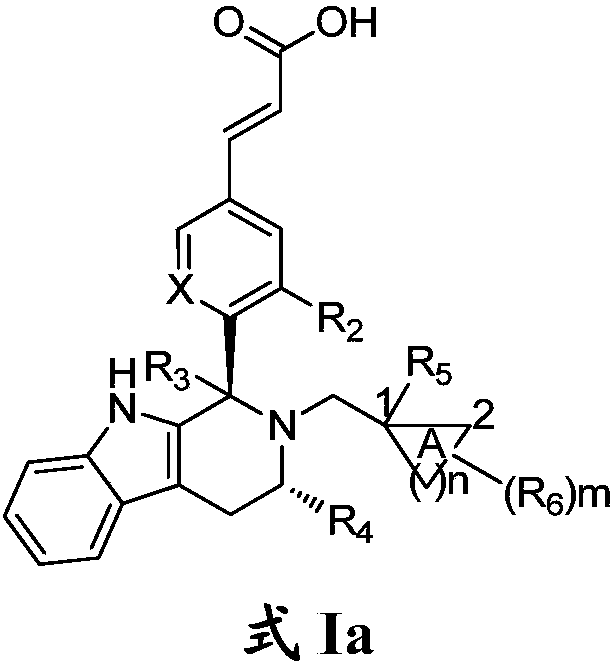
3-substituted acrylic acid compound as well as preparation method and application thereof
A compound and hydrate technology, applied in the field of medicine, can solve the problems of low efficacy and poor drug characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
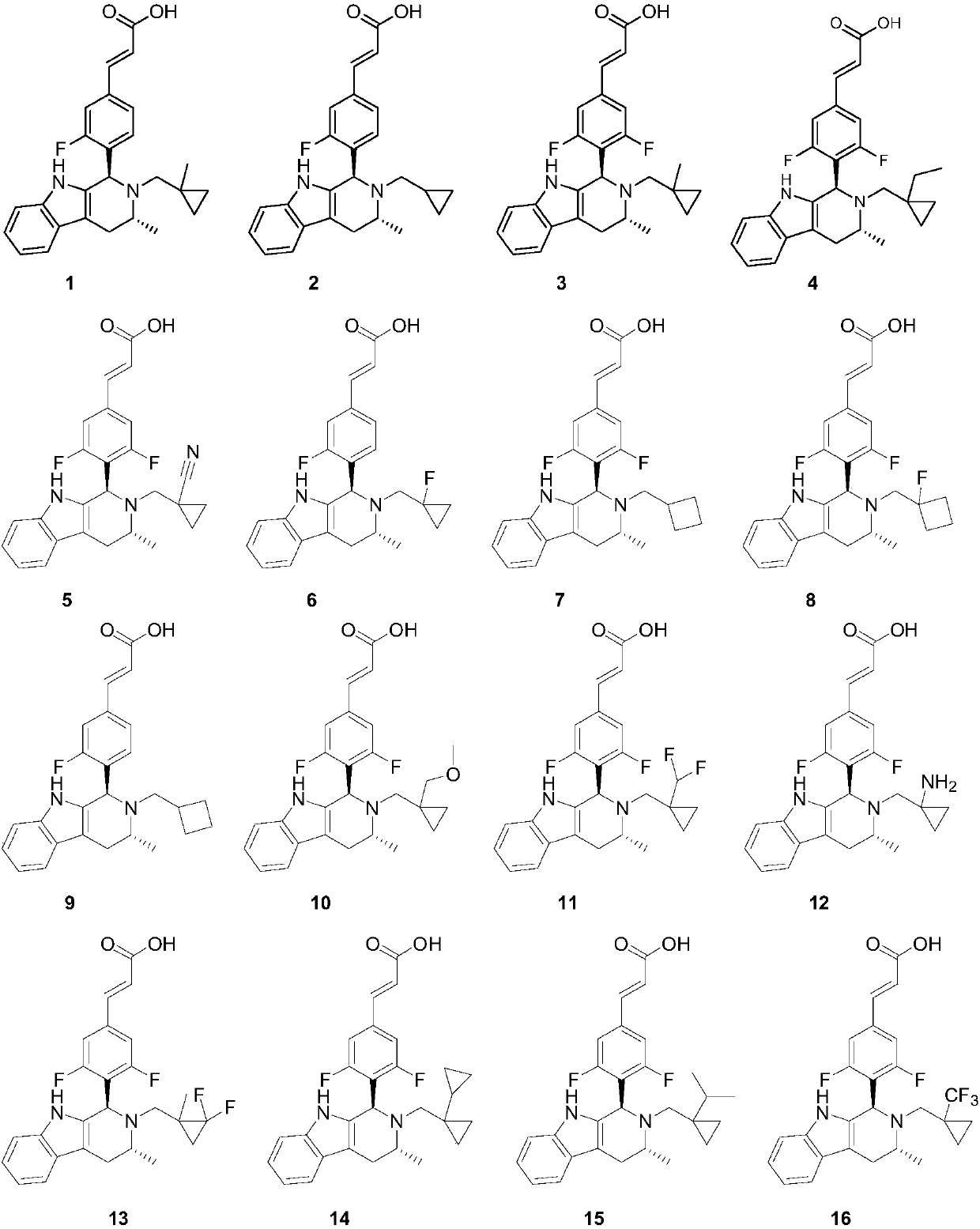
[0135] (E)-3-(3-fluoro-4-((1R,3R)-3-methyl-2-((1-methylcyclopropyl)methyl)-2,3,4,9-tetra Hydrogen-1H-pyrido[3,4-b]indol-1-yl)phenyl)acrylic acid (Compound 1)
[0136] Reaction route:
[0137]
[0138] The first step: (E)-3-(3-fluoro-4-formylphenyl)methyl acrylate (compound 1-2)
[0139] To a solution of 2-fluoro-4-bromobenzaldehyde (2.019 g, 10 mmol) and potassium carbonate (2.76 g, 20 mmol) in DMF (50 mL) was added palladium acetate (224 mg, 1 mmol), triphenylphosphine (1.048g, 4mmol) and methyl acrylate (1.6g, 20mmol), and stirred at 80°C for 12h. The post-processing crude product was subjected to silica gel column chromatography (EA:PE=5:1) to obtain compound 1-2 (2 g, yield 96%).
[0140] MS m / z(ESI): 209[M+H] + .
[0141] The second step: (1-methylcyclopropyl) formaldehyde (compound 1-4)
[0142] To a suspension of (1-methylcyclopropyl)methanol (500mg, 5.8mmol) and silica gel (500mg) in DCM (20mL) was added pyridinium chlorochromate (1.249g, 5.8mmol), then stirre...
Embodiment 2
[0154] (E)-3-(4-((1R,3R)-2-(cyclopropylmethyl)-3-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4 -b] indol-1-yl)-3-fluorophenyl)acrylic acid (compound 2)
[0155] Reaction route:
[0156]
[0157] Using a method similar to Example 1, replacing (1-methylcyclopropyl)methanol in Example 1 with cyclopropylmethanol, Compound 2 was obtained.
[0158] MS m / z(ESI):405[M+H] + .
[0159] 1 HNMR: (400MHz, CD 3 OD)δ: 7.58(d,J=16.0Hz,1H),7.51-7.47(m,2H),7.36–7.24(m,2H),7.14–7.01(m,3H),6.55(d,J=16.0 Hz,1H),5.75(s,1H),3.83(dd,J=12.1,6.8Hz,1H),3.15-3.08(m,1H),2.91(d,J=12.5Hz,1H),2.74(dd ,J=15.9,7.4Hz,1H),2.39(d,J=13.1Hz,1H),1.10-1.15(m,4H),0.54–0.44(m,2H),0.45–0.31(m,2H).
Embodiment 3
[0161] (E)-3-(3,5-difluoro-4-((1R,3R)-3-methyl-2-((1-methylcyclopropyl)methyl)-2,3,4, 9-tetrahydro-1H-pyrido[3,4-b]indol-1-yl)phenyl)acrylic acid (Compound 3)
[0162]
[0163] Using a method substantially similar to Example 1, substituting 2,6-difluoro-4-bromobenzaldehyde for 2-fluoro-4-bromobenzaldehyde in Example 1, Compound 3 was obtained.
[0164] MS m / z(ESI):437[M+H] + .
[0165] 1 H NMR (400MHz, CD 3 OD) δ7.57(d, J=16.0Hz, 1H), 7.42(dd, J=6.9, 1.1Hz, 1H), 7.24–7.18(m, 3H), 7.04-6.95(m, 2H), 6.55( d,J=16.0Hz,1H),5.37(s,1H),3.87(dd,J=11.1,4.8Hz,1H),3.08(dd,J=15.1,3.6Hz,1H),2.68(dd,J =15.0,3.2Hz,1H),2.48(dd,J=27.8,12.9Hz,2H),1.31–1.29(m,1H),1.11(d,J=6.5Hz,2H),1.00(s,3H) ,0.42–0.33(m,2H),0.27-0.16(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com