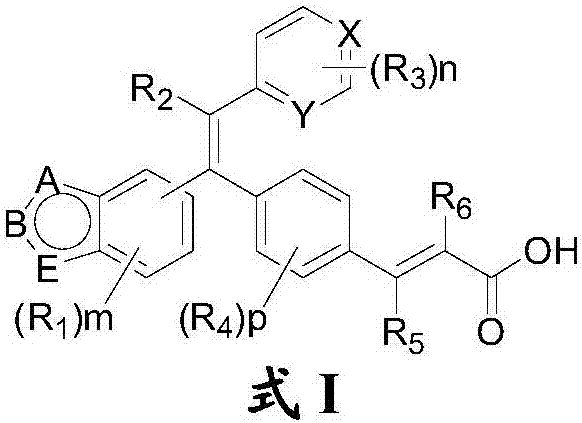
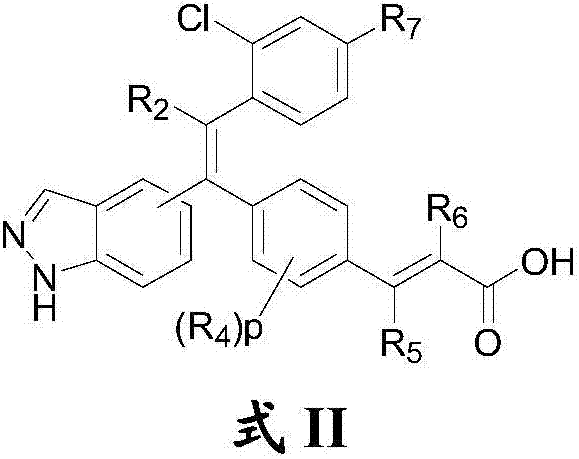
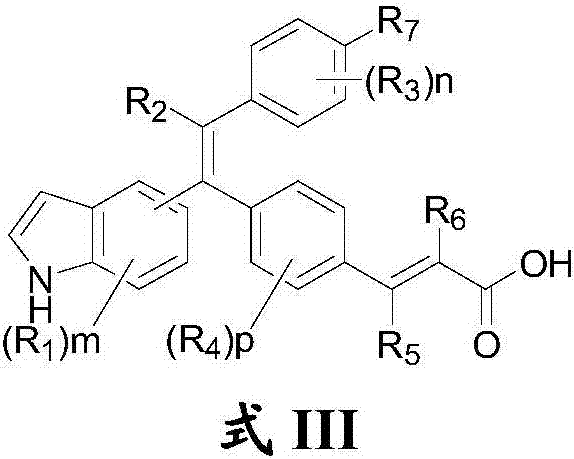
Substituted olefin compound, and preparation method and application thereof
A compound and solvate technology, applied in the field of medicine, can solve the problems of poor drug characteristics and low efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] Example 1: (E)-3-(4-((E)-2-(3-chloropyridin-4-yl)-1-(1H-indol-4-yl)-1-butenyl) Phenyl)acrylic acid (Compound 1)
[0180] Reaction route:
[0181]
[0182] The first step: N-acetyl-4-bromo-indole (compound 1-2)
[0183] Under nitrogen atmosphere, DMAP (122 mg, 1 mmol) was added to a suspension of 4-bromoindole (1.96 g, 10 mmol) in acetic anhydride (50 mL), and the reaction mixture was heated to about 140°C for about 3-4 hours. The reaction mixture was cooled to room temperature, poured into ice water, extracted with ethyl acetate, the organic phases were combined, washed with saturated aqueous sodium bicarbonate solution, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure to obtain an oil Purified by column chromatography (PE:EA=10:1) to obtain N-acetyl-4-bromo-indole (2.2 g, yield 92%).
[0184] MS m / z(ESI):238[M+H] + .
[0185] The second step: N-acetyl-4-butynyl-indole (compound 1-4...
Embodiment 2
[0204] Example 2: (E)-3-(4-((Z)-2-(2-chlorophenyl)-1-(1H-indazol-5-yl)-1-butenyl)-3- Fluorophenyl)acrylic acid (Compound 2)
[0205] Reaction route:
[0206]
[0207]The first step: 5-bromo-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
[0208] To a solution of 5-bromoindazole (1.97 g, 10 mmol) in dichloromethane (50 mL) under nitrogen atmosphere, were added 3,4-dihydro-2H-pyran (1.26 g, 15 mmol) and p-toluenesulfonic acid ( 194mg, 1mmol), the reaction mixture was reacted at room temperature for about 11-12h. Workup gave 5-bromo-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole (2.52 g, yield: 90%).
[0209] MS m / z(ESI):281[M+H] + .
[0210] The second step: 5-(1-butynyl)-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
[0211] Under nitrogen atmosphere, to 5-bromo-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole (2.5g, 8.9mmol) in N,N-dimethylacetamide (45mL) solution In, add cesium carbonate (5.8g, 17.8mmol), dppf (500mg, 0.9mmol), cuprous iodide (171mg, 0.9mmol), palladium acetate (200mg, 0.9...
Embodiment 3
[0232] Example 3: (E)-3-(4-((3E)-2-(2-chloro-4-fluorophenyl)-1-(1H-indol-4-yl)-1-butenyl ) phenyl) acrylic acid (Compound 3)
[0233]
[0234] The first step: tert-butyl 4-bromo-1H-indolyl-1-carbonate
[0235] To a solution of 4-bromoindole (1.96g, 10mmol) in dichloromethane (50mL), DMAP (122mg, 1mmol) and di-tert-butyl dicarbonate (2.4g, 11mmol) were added, and the reaction system was stirred at room temperature for 8h, then Work-up gave tert-butyl 4-bromo-1H-indolyl-1-carbonate (2.96 g, 99% yield).
[0236] MS m / z(ESI):241[M-56+1] + .
[0237] The second step: tert-butyl 4-(1-butynyl)-1H-indole-1-carbonate
[0238] The operation is the same as the second step in Example 2, replacing 5-bromo-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole with 4-bromo-1H-indolyl-1 - tert-butyl carbonate to give tert-butyl 4-(1-butynyl)-1H-indole-1-carbonate (2.15 g, yield 80%).
[0239] MS m / z(ESI):214[M-56+1] + .
[0240] The third step: (Z)-4-(1,2-diboronic acid pinacol ester-1-butenyl)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com