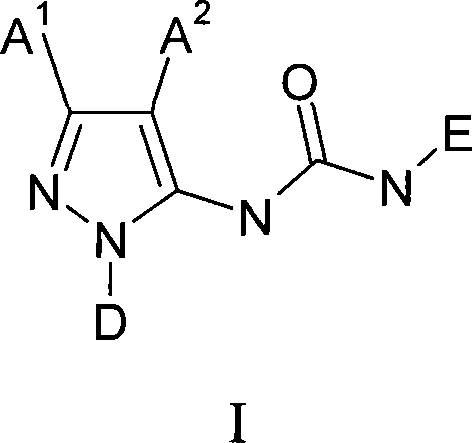
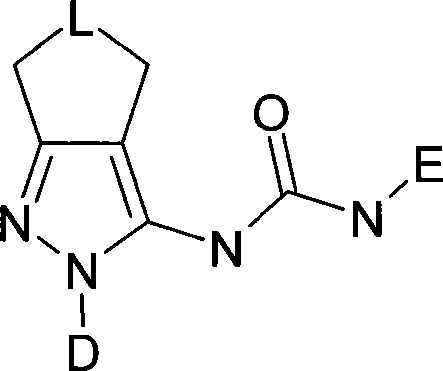
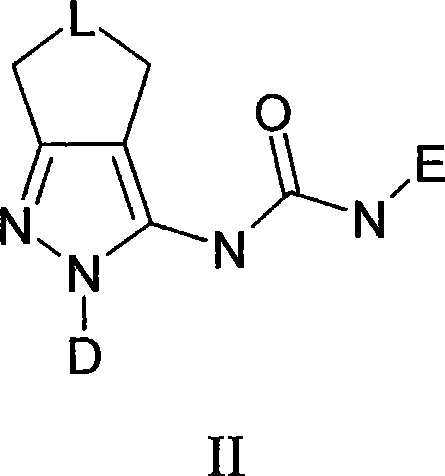
Novel pyrazole derivatives and their use as modulators of nicotinic acetylcholine receptors
A technology of precursors and enantiomers, applied in the field of compounds or their pharmaceutically acceptable salts, can solve problems such as limiting the duration of agonist action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0165] Example 1: 1-(5-Cyclopropyl-2-phenyl-2H-pyrazol-3-yl)-3-(4-ethoxyphenyl)urea
[0166]
[0167] A solution of 5-cyclopropyl-2-phenyl-2H-pyrazol-3-ylamine (60mg, 0.30mmol) and 4-ethoxyphenylisocyanate (49mg, 0.30mmol) in dichloromethane (5mL) After stirring at 70 °C for 2 h, the solvent was evaporated. The resulting residue was triturated with dichloromethane / hexane (1:2, 40 mL) overnight. The solid was collected by filtration, washed with dichloromethane / hexane (1:2, 3X), and air dried to give 1-(5-cyclopropyl-2-phenyl-2H-pyrazol-3-yl)- 3-(4-Ethoxyphenyl)urea (82 mg, 75%) as a white solid. MS (APCI+) 363 [M+1]+. 1 H-NMR (300MHz, d 6 -DMSO): δ8.74 (1H, s), 8.27 (1H, s), 7.58-7.46 (4H, m), 7.44-7.35 (1H, m), 7.27 (2H, d, J=9.0), 6.83 (2H, d, J = 9.0), 6.14 (1H, s), 3.96 (2H, q, J = 7.0), 1.94-1.80 (1H, m), 1.29 (3H, t, J = 7.0), 0.93- 0.83 (2H, m), 0.74-0.62 (2H, m).
Embodiment 2
[0168] Example 2: 1-(4-methylphenyl)-3-(2-phenyl-2,6-dihydro-4H-thieno[3,4-c]pyrazol-3-yl)urea
[0169]
[0170] 2-Phenyl-2,6-dihydro-4H-thieno[3,4-c]pyrazol-3-ylamine (60mg, 0.28mmol) and 4-methylphenylisocyanate (38mg, 0.28mmol ) in dichloromethane (5 mL) was stirred at 60 °C for 2 h, and the solvent was evaporated. The resulting residue was triturated with dichloromethane / hexane (1:2, 40 mL) overnight. The solid was collected by filtration, washed with dichloromethane / hexane (1:2, 3X), and air dried to give 1-(4-methylphenyl)-3-(2-phenyl-2,6-di Hydrogen-4H-thieno[3,4-c]pyrazol-3-yl)urea (32 mg, 32%) as a beige solid. MS (APCI+) 355 [M+1]+. 1 H-NMR (300MHz, d 6 -DMSO): δ8.81 (1H, s), 8.37 (1H, s), 7.58-7.47 (4H, m), 7.47-7.36 (1H, m), 7.28 (2H, d, J=8.1), 7.07 (2H, d, J = 8.1), 3.95 (2H, s), 3.89 (2H, s), 2.23 (3H, s).
[0171] The following compounds were prepared in substantially a similar manner to Example 1 or Example 2 using the appropriate amine and isocyana...
Embodiment 3
[0172] Example 3: 1-(4-Methoxy-phenyl)-3-(2-phenyl-2,6-dihydro-4H-thieno[3,4-c]pyrazol-3-yl)-urea
[0173]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com