Method for producing hydrogen peroxide
一种过氧化氢、制造方法的技术,应用在过氧化氢、化学仪器和方法、过氧化物/过氧水合物/过氧酸/超氧化物/臭氧化物等方向,能够解决增大设备费用负担、金属类从催化剂溶出量增加、增加催化剂初期投资等问题,达到杂质含量少的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
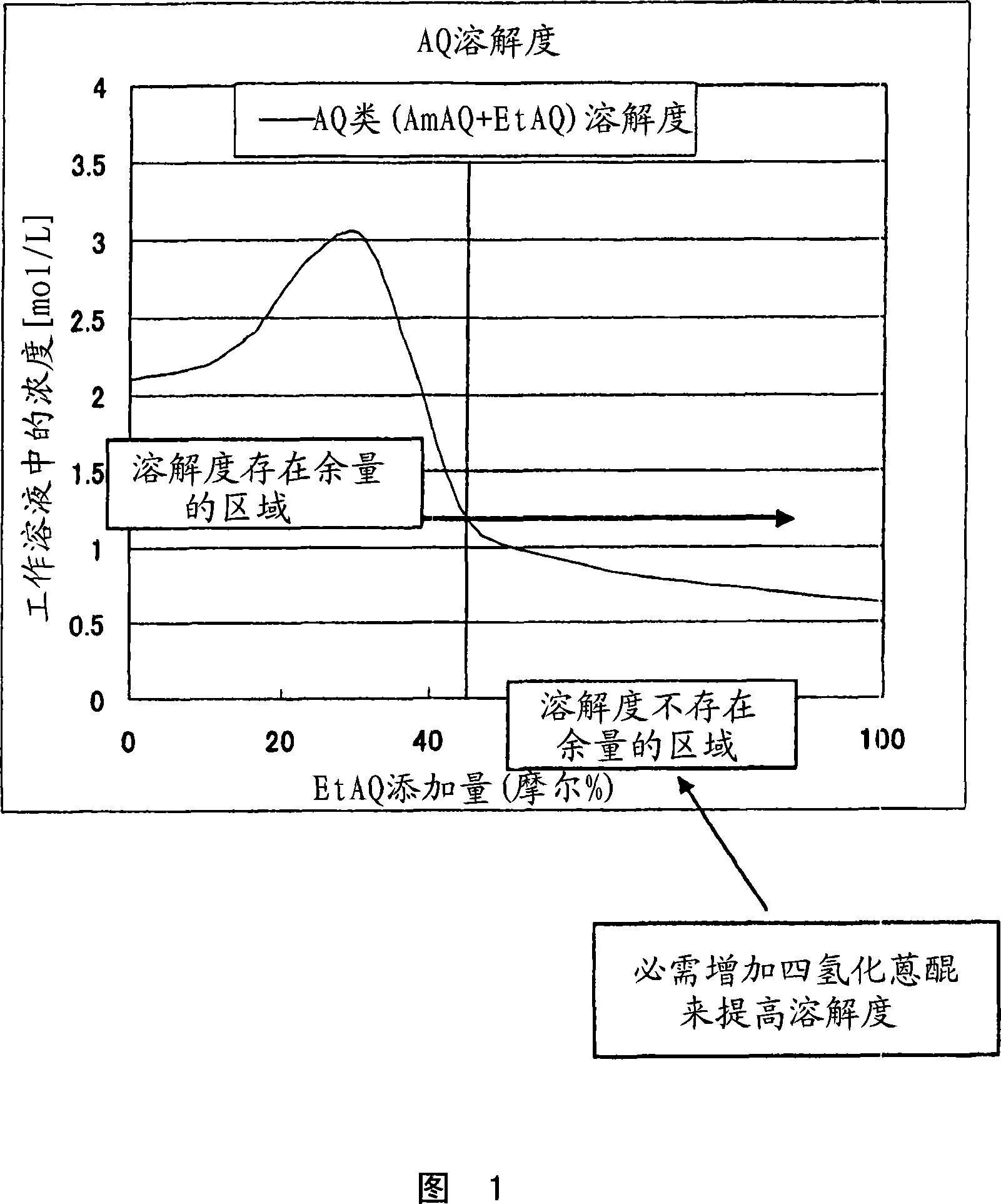
Image
Examples
Embodiment 1
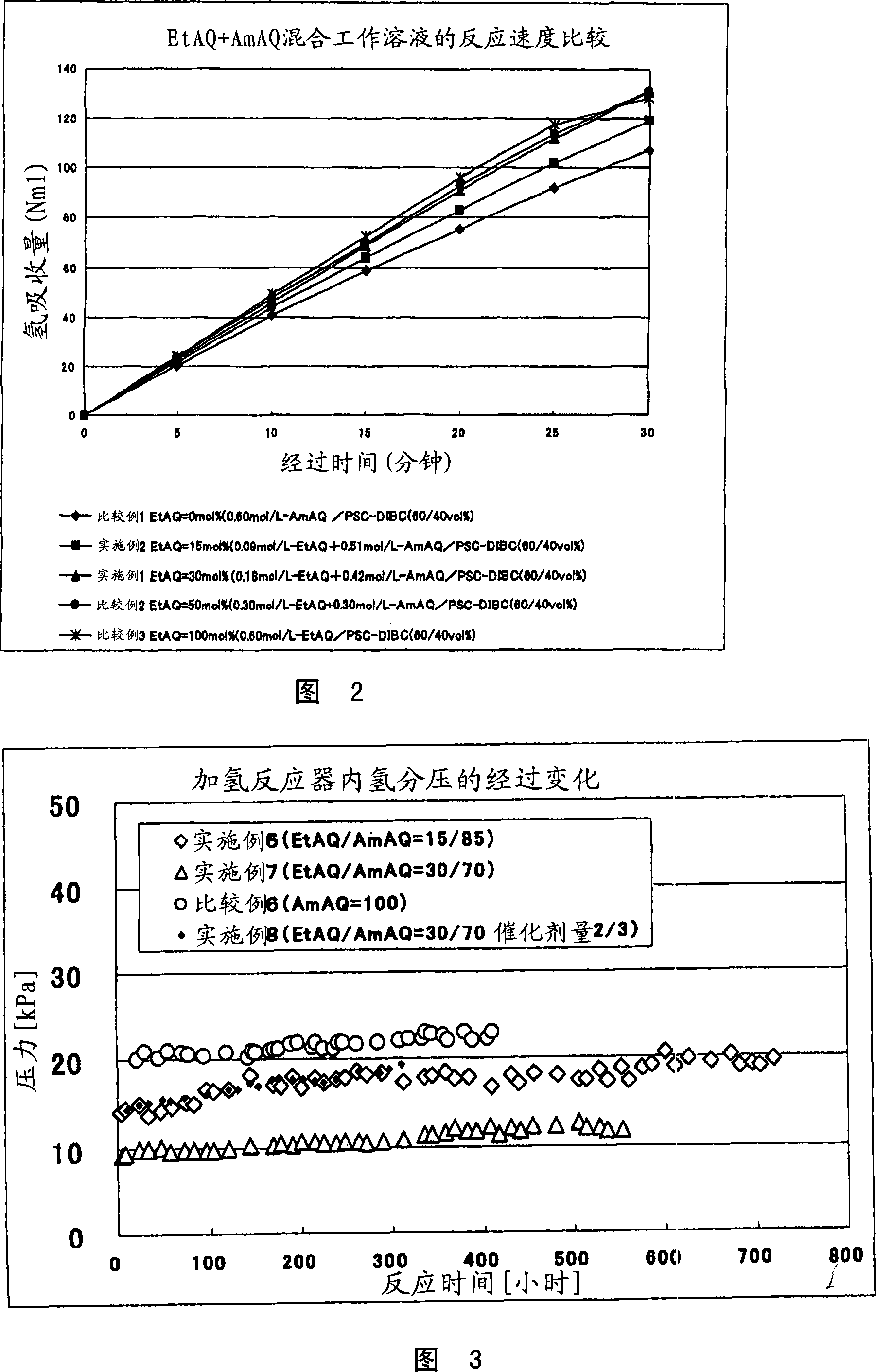
[0042] With the anthraquinone concentration in the working solution as 0.6 mol / L and the composition ratio as EtAQ (30 mol%)+AmAQ (70 mol%), the hydrogen absorption in the working solution was evaluated.
[0043] The specific measurement method is as follows. 100 mg of supported palladium catalyst (equivalent to Pd=1 mg) and 25 ml of working solution were added to a 100 ml three-necked flask. As a solvent for the working solution, a mixed solvent of 60% by volume of pseudocumene and 40% by volume of diisobutylmethanol was used. A flask equipped with a magnetic induction stirrer and a vacuum piston that can be completely sealed inside is set in an atmospheric pressure hydrogenation reaction device. This device detects the pressure change in the flask through the water level, and supplies hydrogen in balance with the hydrogen absorption from the metering tube through the switching solenoid valve. The hydrogen metering tube includes a burette part and a water storage part, and ...
Embodiment 2
[0045] The hydrogen absorption rate was measured using the same method as in Example 1 except that EtAQ (15 mol%)+AmAQ (85 mol%) was used. The hydrogen absorption rate was 119 Nml. The results of the hydrogen absorption rate are shown in FIG. 2 .
Embodiment 3
[0054] Using a hydrogen peroxide production device that has a hydrogenation process, an oxidation process, an extraction process, and a regeneration process, the working solution is circulated to study the components of the produced hydrogen peroxide.
[0055] The structure of the hydrogen peroxide production apparatus is as follows.
[0056] Hydrogenation process: Stirred hydrogenation reactor, hydrogenation catalyst is Pd catalyst;
[0057] Oxidation process: in the form of a multi-stage counter-flow oxidation tower (reaction temperature: 40°C).
[0058] The working solution is a substance that is used for many years, and its composition contains age-degraded substances that cannot be analyzed. The main composition of anthraquinones in the working solution and the phthalic acid content in the resulting hydrogen peroxide are shown below.
[0059] Anthraquinones in working solution
[0060] Amyl anthraquinone=420mmol / l; Amyl tetrahydroanthraquinone=80mmol / l;
[0061] Ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com