Method for preparing fluticasone propionate
A kind of technology of fluticasone propionate, equation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
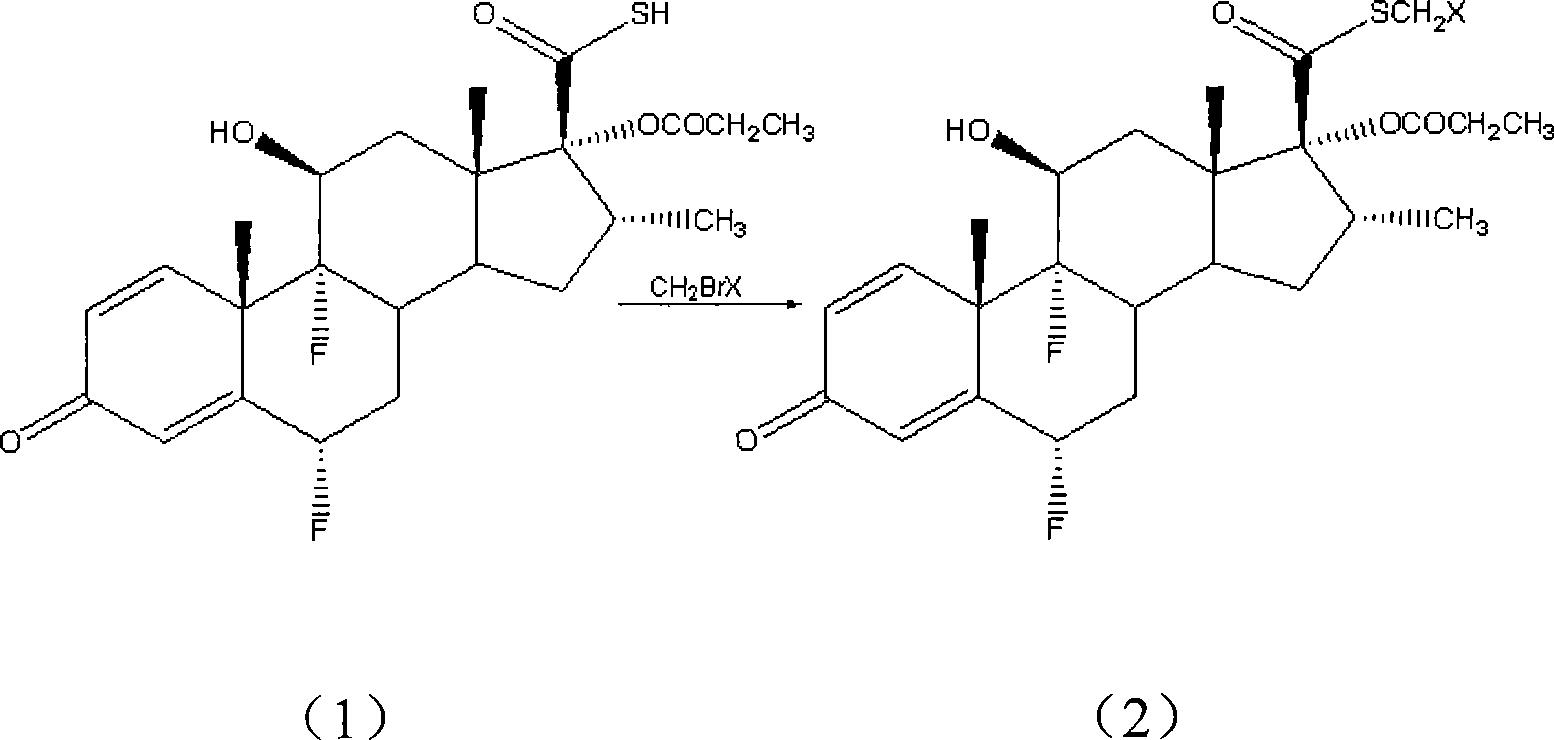
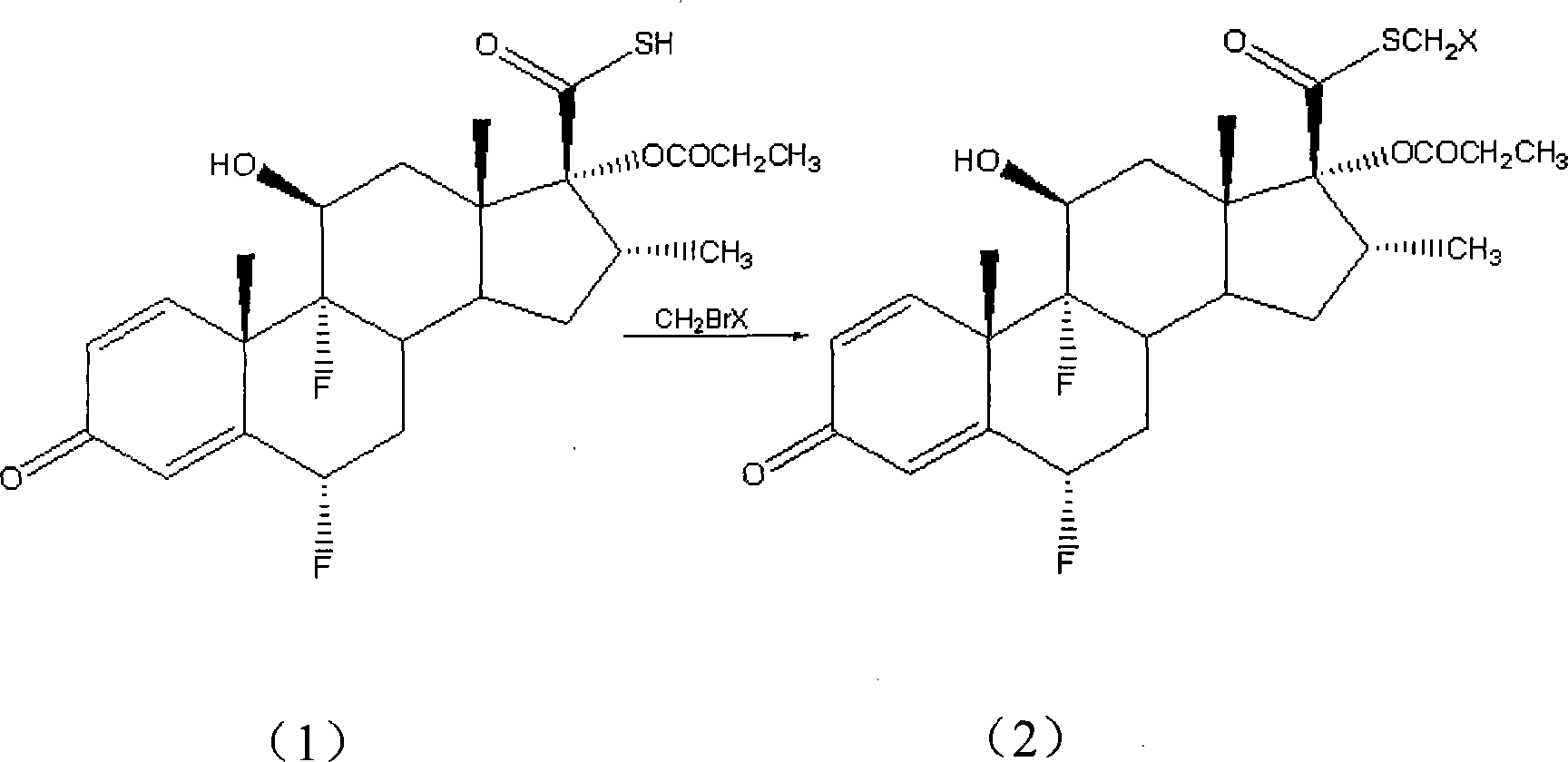
[0031] S-(chloromethyl)-6α,9α-difluoro-11β-hydroxy-16α-methyl-17α-propionyloxy-3-ketoandrost-1,4-diene-17β-thiocarboxylic acid Preparation of esters:
[0032] Add 6α, 9α-difluoro-11β-hydroxyl-16α-methyl-17α-propionyloxy-3-ketoandrost-1,4-diene-17β-thiocarboxylic acid in a 250mL three-neck flask (Fw: 468.55, 10.0g, 21.3mmol), DMF100mL, sodium bicarbonate (Fw: 84.01, 7.2g, 85.2mmol), then chlorobromomethane (Fw: 129.38, 11.0g, 85.2mmol) was added dropwise to the system at room temperature In 30min, the dropwise addition was completed, and then the system continued to stir and react at room temperature for 2h. After the reaction, the mixture in the system was poured into 500mL of water, a large amount of white solid was precipitated, filtered, the filter cake was fully washed with distilled water, and dried to obtain S-(chloromethyl)-6α,9α-difluoro-11β-hydroxyl-16α- Methyl-17α-propionyloxy-3-ketoandrost-1,4-diene-17β-thiocarboxylate (Fw: 517.03, 10.5 g, yield: 95%). HPLC conte...
Embodiment 2
[0034] S-(bromomethyl)-6α,9α-difluoro-11β-hydroxy-16α-methyl-17α-propionyloxy-3-ketoandrost-1,4-diene-17β-thiocarboxylic acid Preparation of esters:
[0035] In a 250 mL three-necked flask, 100 mL of acetone and potassium carbonate (Fw: 138.21, 8.8 g, 63.9 mmol) were sequentially added, and then dibromomethane (Fw: 173.83, 14.8 g, 85.2 mmol) was added to the system. 6α,9α-difluoro-11β-hydroxy-16α-methyl-17α-propionyloxy-3-ketoandrost-1,4-diene-17β-thiocarboxylic acid (Fw: 468.55, 10.0g, 21.3mmol) was dissolved in 50mL of acetone, and was added dropwise to the system at room temperature, and the dropwise addition was completed in about 30min, and then the system was stirred and reacted at room temperature for 4h. Warming to reflux for 1h. After the reaction was completed, the system was cooled to room temperature, most of the solvent was evaporated under reduced pressure, the mixture was poured into 300mL water, a large amount of white solid was precipitated, filtered, the fi...
Embodiment 3
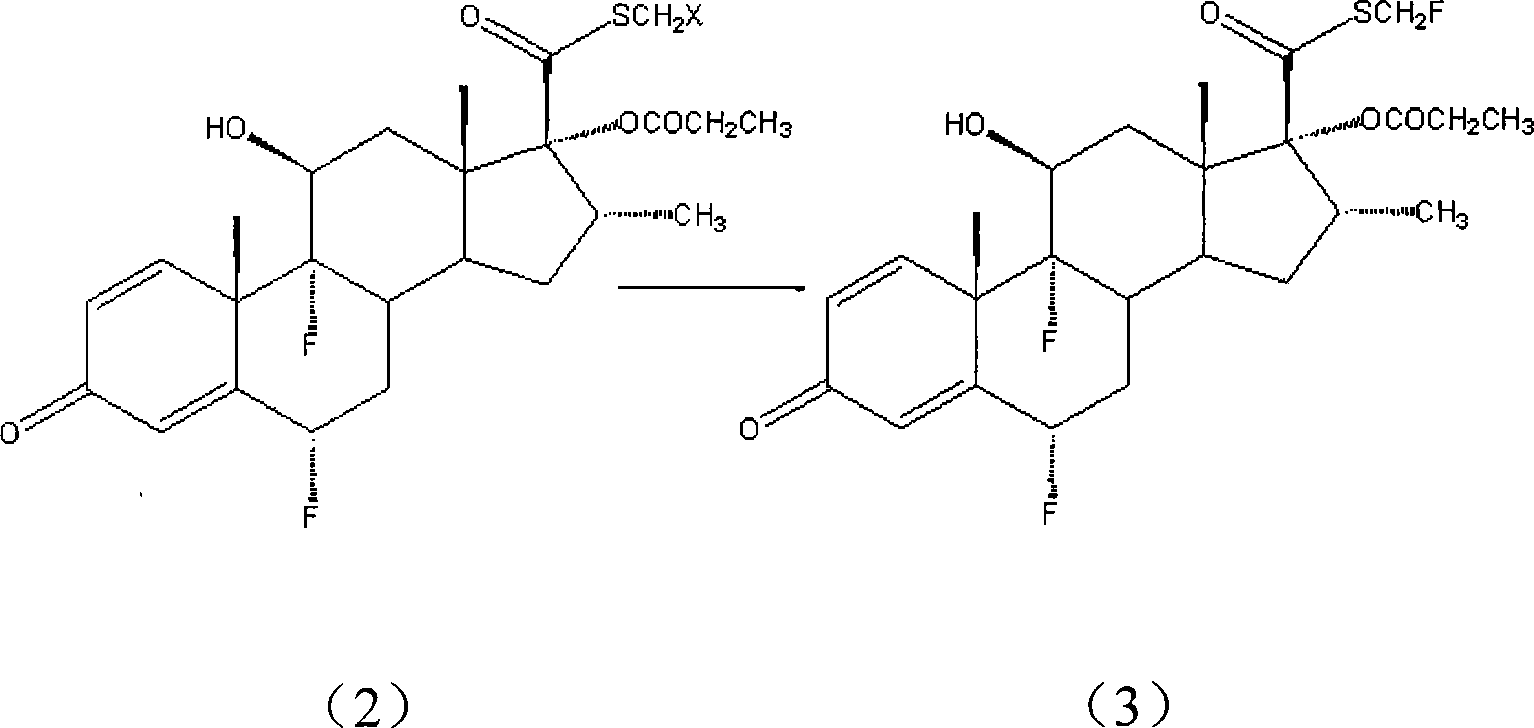
[0037] S-(fluoromethyl)-6α,9α-difluoro-11β-hydroxy-16α-methyl-17α-propionyloxy-3-ketoandrost-1,4-diene-17β-thiocarboxylic acid Preparation of esters:
[0038] Anhydrous tetrabutylammonium fluoride (Fw: 261.46; 5.07g; 19.4mmol) was successively added into a 100mL dry three-necked flask, followed by [Bmim][BF 4] 20.0mL was added to the system and stirred for 5min. The S-(chloromethyl)-6α,9α-difluoro-11β-hydroxyl-16α-methyl-17α-propionyloxy-3-ketoandrost-1,4-diene- 17β-thiocarboxylic acid (Fw: 517.03, 5.0 g, 9.7 mmol) was added to the system, and then 50 mL of anhydrous acetonitrile was added to the system. The system was heated to reflux for 15h. After the reaction was completed, the system was cooled to room temperature, most of the solvent was evaporated under reduced pressure, the mixture was poured into 150mL water, a large amount of white solid was precipitated, filtered, the filter cake was washed with distilled water, dried, and then recrystallized from acetone to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com