Aryl substituted imidazoline-2-ketones derivant, producing method and uses of the same
An imidazoline and derivative technology, applied in the field of compound preparation, can solve the problems of poor water solubility, weak antitumor activity, unstable cis-stilbene structure, etc., and achieves the effects of reasonable design and simple preparation method
Inactive Publication Date: 2010-11-17
ZHEJIANG UNIV
View PDF1 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Although CA-4 has cytotoxic activity and anti-tubulin polymerization activity in vitro, CA-4, as an anti-mitotic agent, only shows weak anti-tumor activity in vivo activity research, the main reason is its poor water solubility, The structure of cis-stilbene is unstable, and it is easy to isomerize into trans configuration
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
specific Embodiment approach
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
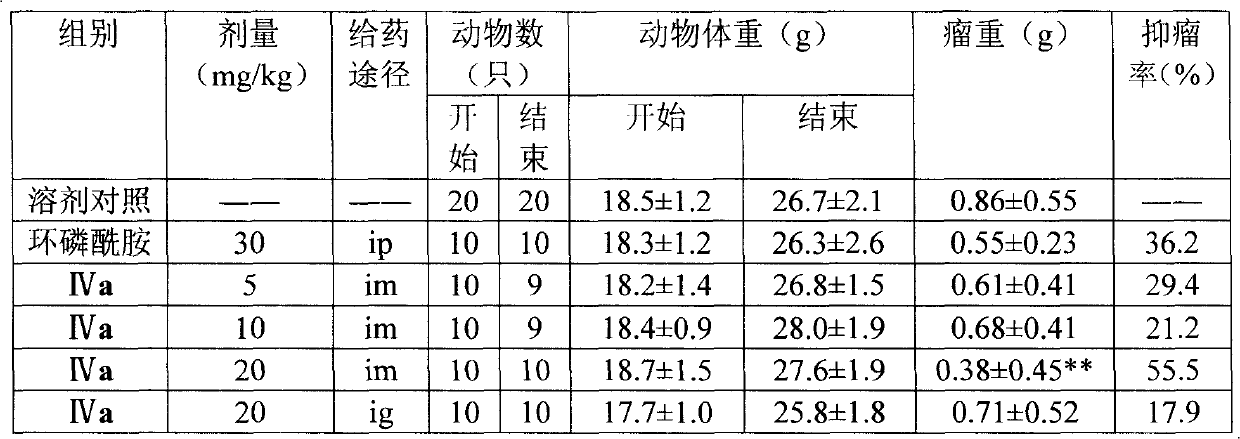
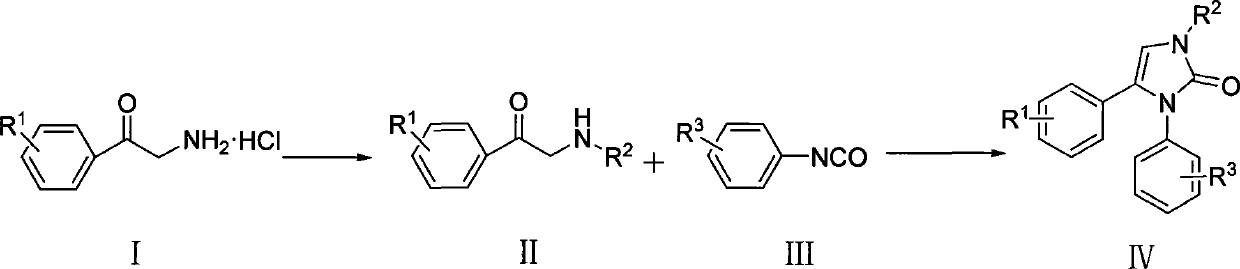
The present invention provides a category of aryl-substituted imidazoline-2-ketone derivatives, a compound with a natural product CombretastatinA-4 as a precursor. The imidazoline-2-ketone five-heterocycle is used to substitute ethylene chain to prepare a series of imidazolinone derivative. The imidazolinone derivative is a compound of novel structure. The initial screening tests of pharmacological activity have proved that a plurality of compounds have inhibitory effects on tumor cells in vitro; part of the compounds have significant inhibitory effects; the IC50 has achieved to Mu M level; wherein, the representative compound IVa has significant anti-tumor activity in vivo. The aryl-substituted imidazoline-2-ketone derivatives provided by the present invention and the related salts can be used in preparation of anti-tumor drugs. The present invention is reasonable in design, simple in preparation method and applicable to practical use. The structure of the present invention is the above formula.
Description
technical field The invention belongs to compound preparation, and mainly relates to the preparation method and application of aryl-substituted imidazolin-2-one derivatives. Background technique The mortality rate caused by malignant tumors ranks second among all diseases, second only to cardiovascular and cerebrovascular diseases. However, clinically used antineoplastic drugs generally have disadvantages such as high toxicity and side effects, and easy drug resistance. Therefore, finding novel drugs with better curative effect, lower toxicity and side effects, and wider anti-tumor spectrum has always been the direction of efforts of medicinal chemists. Combretastatin A-4 (CA-4) is the most effective anti-mitotic natural product isolated from the plant Combretum Caffrum. The compound has good cytotoxic activity on various human cancer cells, including multi-drug resistant cancer cell lines. Due to its simplicity in structure and good activity in anti-tumor, including dru...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D233/70A61K31/4174A61P35/00
Inventor 胡永洲薛娜杨晓春杨波何俏军
Owner ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com