2,8,9-trisubstituted-9h-purine compound and its salt and application
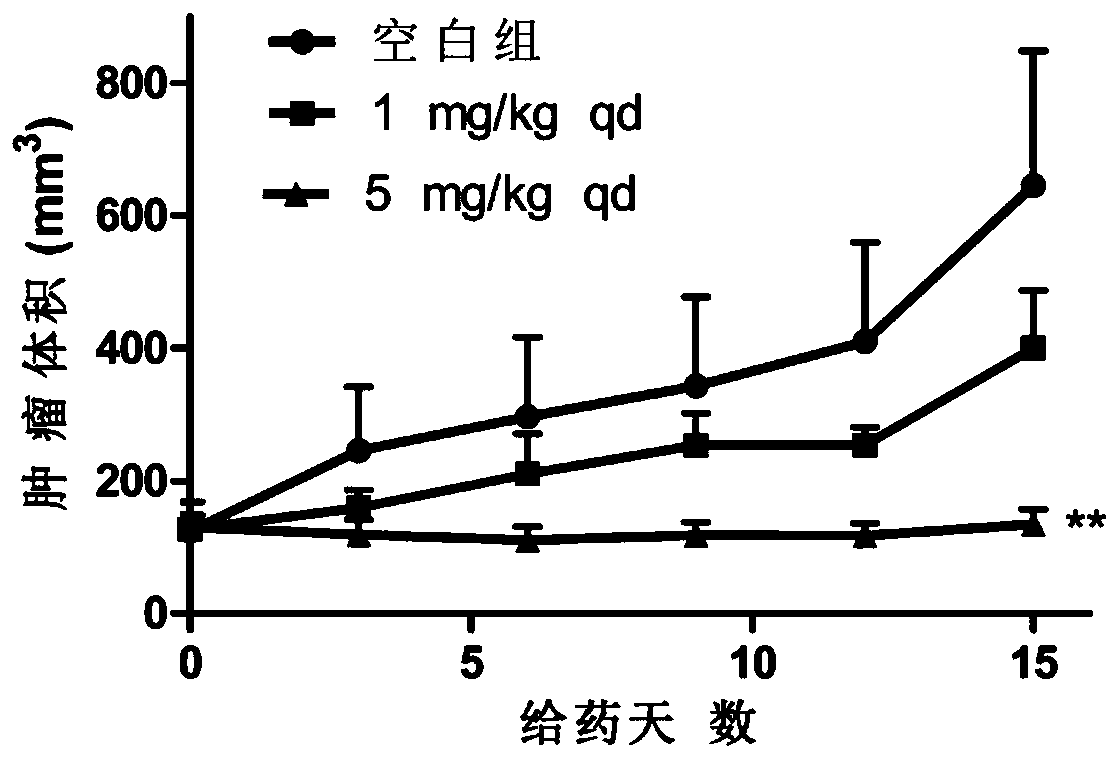
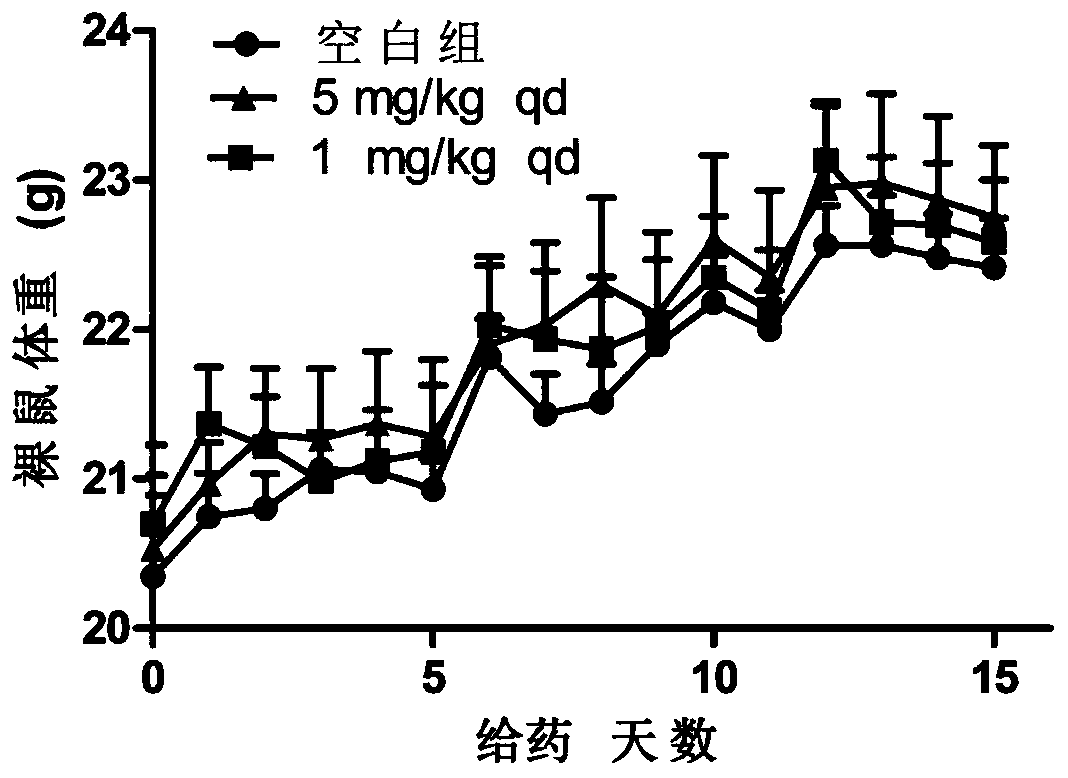
A technology of purines and compounds, applied in the field of anticancer drugs, can solve the problem of decreased effect of osimertinib and achieve significant antitumor activity in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
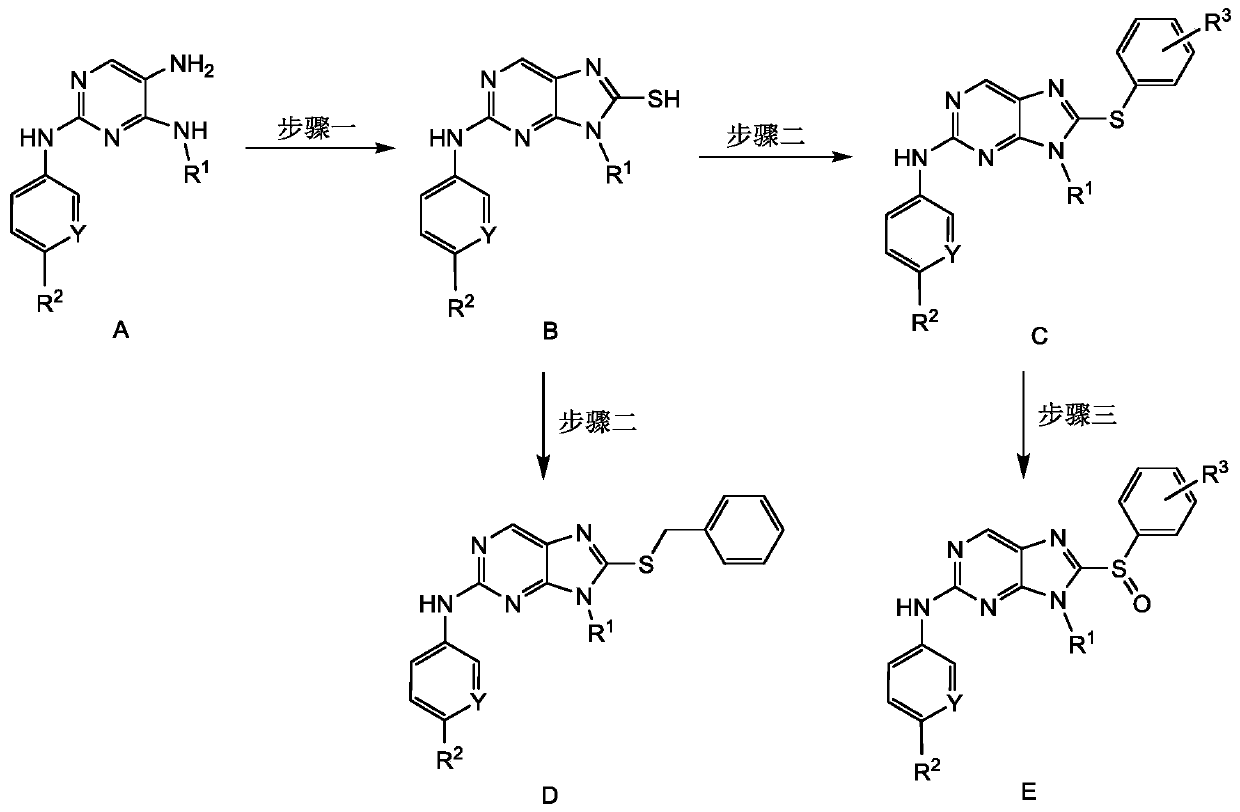
[0034] The synthetic method of 2,8,9-trisubstituted-9H-purine compounds, the synthetic route is as follows figure 1 shown, including the following steps:
[0035] Step 1: Reference (J.Med.Chem.2012,55,10685-10699) to synthesize compound A, A was refluxed together with carbon disulfide and potassium hydroxide in ethanol / water (10:1) to obtain intermediate B;
[0036] Step 2: Under the catalysis of ketone iodide, intermediate B and iodobenzene were stirred in dimethyl sulfoxide at 140°C for 24 hours to obtain product C, namely 8-phenylthio-2,9-disubstituted-9H -purine compounds; or, in the presence of potassium carbonate, intermediate B and benzyl bromide are stirred and refluxed in acetone for 4h to obtain product D, that is, 8-benzylthio-2,9-disubstituted-9H-purines compound;
[0037] Step 3: C is oxidized by m-chloroperoxybenzoic acid in dichloromethane to obtain product D, namely 8-benzenesulfinyl-2,9-disubstituted-9H-purine compound.
Embodiment 1
[0039] Example 1: 2-(4-(4-methyl-1-piperazinyl)anilino)-8-phenylthio-9-(2H-4-tetrahydropyranyl)-9H-purine (structural formula 1) Synthesis of:
[0040] Step 1: Synthesis of 2-(4-(4-methyl-1-piperazinyl)anilino)-8-mercapto-9-(2H-4-tetrahydropyranyl)-9H-purine (B1)
[0041]
[0042] Reference J. Med. Chem. 2012, 55, 10685-10699 to prepare A1. A1 (0.40g), carbon disulfide (95mg), potassium hydroxide (70mg), ethanol (20mL) and water (2mL) were added to a 100mL eggplant-shaped bottle, the mixture was heated to reflux for 3h, the solvent was removed under reduced pressure, and the residue was subjected to silica gel column chromatography Separation (dichloromethane:methanol=20:1) gave intermediate B1 (0.40g) with a yield of 90.3%.
[0043] Step 2: 2-(4-(4-methyl-1-piperazinyl)anilino)-8-phenylthio-9-(2H-4-tetrahydropyranyl)-9H-purine (structural formula 1 )Synthesis
[0044]
[0045]Add B1 (0.20 g), iodobenzene (80 μL), cuprous iodide (6.7 mg), potassium carbonate (0.20 g)...
Embodiment 2
[0046] Example 2: 2-(4-(N-methyl-2-dimethylaminoethylamino)anilino)-8-phenylthio-9-(2H-4-tetrahydropyranyl)-9H-purine (Structural formula 2) synthesis:
[0047]
[0048] Reference J. Med. Chem. 2012, 55, 10685-10699 to prepare A2. The synthesis of B2 is the same as that of B1. Compound 2 was synthesized by replacing B1 with B2, and the process was the same as that of compound 1. Yield 55.3%. 1 H NMR (400MHz, CDCl 3 )δ8.69(s,1H,Ar-H),7.52(s,1H,Ar-H),7.50(d,J=2.3Hz,2H,Ar-H),7.49–7.47(m,1H,Ar-H) -H),7.41–7.34(m,3H,Ar-H),6.77(d,J=9.0Hz,2H,Ar-H),4.71–4.62(m,1H,CH),4.11(dd,J= 11.5, 4.2Hz, 2H, CH×2), 3.48–3.45(m, 2H, CH 2 ), 3.42(d, J=11.7Hz, 2H, CH×2), 2.96(s, 3H, CH 3 ),2.95–2.86(m,2H,),2.55–2.47(m,2H,CH 2 ),2.31(s,6H,CH 3 ×2), 1.55(dd, J=12.4, 2.5Hz, 2H, CH×2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com