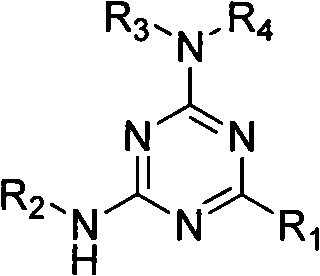
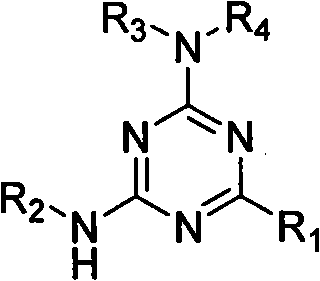
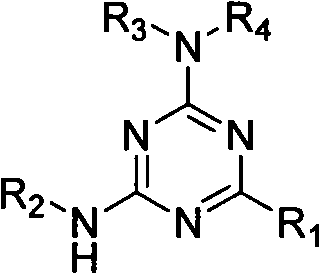
Use of 2-alkyl substituted 4,6-diamino-1,3-5-triazine derivate
A technology of hydrocarbyl substitution and diamino, applied in the application field of 2-hydrocarbyl substitution of 4,6-diamino-1,3,5-triazine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0015] Example: In vitro inhibitory effect of 2-hydrocarbyl substituted 4,6-diamino-1,3,5-triazine derivatives on different tumor cells
[0016] (1) Experimental materials
[0017] Cell lines: human lung cancer A549 cells, human leukemia K562 cells, human prostate cancer PC-3 cells, human ovarian cancer HO8910 cells and human breast cancer MCF-7 cells.
[0018] Medium: RPMI 1640 medium, containing 10% calf serum.
[0019] Drug and preparation: The drug is the 2-hydrocarbyl substituted 4,6-diamino-1,3,5-triazine derivative synthesized above dissolved in dimethyl sulfoxide (DMSO). Compound structures are shown in Table 1. The positive control drug was doxorubicin.
[0020] (2) Experimental method
[0021] The above-mentioned tumor cells in the logarithmic growth phase were divided into 5×10 3 Seed in a 96-well plate at a density of 96 wells, add 200 μl of cell suspension to each well, and add 5 concentrations of 2-hydrocarbyl-substituted 4,6-diamino-1,3,5-triazines after 24...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com