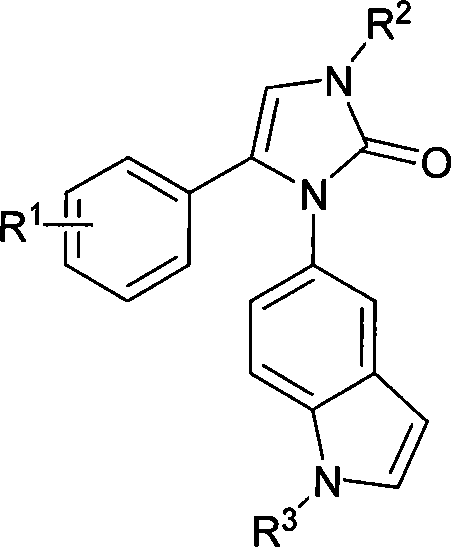
Indole-substituteing imidazoline-2-ketones derivant, preparing method and uses of the same
A technology of imidazoline and derivatives, applied in the field of compound preparation, can solve the problems of unstable cis-stilbene structure, poor water solubility, weak anti-tumor activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
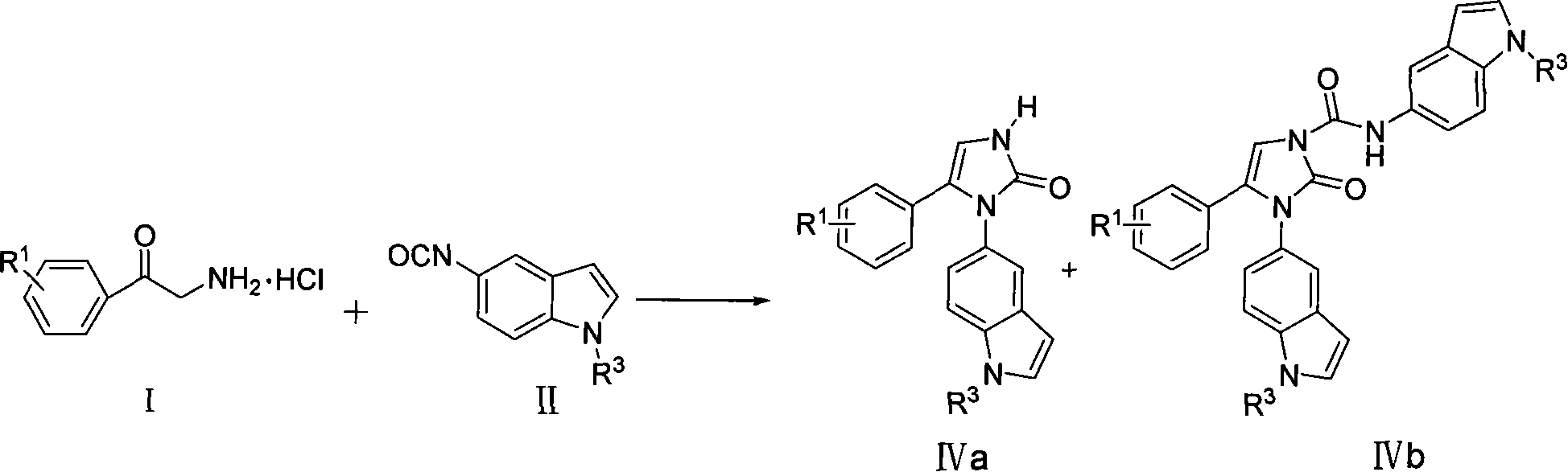
[0025] Example 1: Preparation of 1-(1H-indol-5-yl)-5-(4-bromophenyl)-4-imidazolidin-2-one (compound IVa1)
[0026] Add 0.53g (4mmol) of 5-amino-1H-indole and 4mL of anhydrous toluene to the reaction flask, cool in an ice-salt bath, and slowly drop into a solution prepared from 0.79g of solid phosgene and 6mL of anhydrous toluene. After dropping, continue stirring and reacting for 1 hour under the protection of nitrogen, remove the ice-salt bath, react at room temperature for 30 minutes, slowly heat up to reflux, and after 4 hours, the reaction solution becomes clear, add 4-bromo-α-aminophenyl ethyl Ketone hydrochloride 0.38g (2mmol), added anhydrous toluene 10mL, heated to reflux overnight. Cool, recover the solvent under reduced pressure, wash the residue with chloroform 20mL×2, combine the filtrates, wash with saturated NaCl solution, and wash the organic layer with anhydrous NaCl 2 SO 4 After drying, the solvent was recovered under reduced pressure to obtain a brown solid...
Embodiment 2
[0028] Example 2: N,3-bis(1H-indol-5-yl)-4-(2,4-difluorophenyl)-4-imidazolidin-2-one-1-carboxamide (compound IVb1) preparation of
[0029] Add 0.58g (4mmol) of 5-amino-1H-indole and 4mL of anhydrous toluene to the reaction flask, cool in an ice-salt bath, and slowly drop into a solution prepared from 0.79g of solid phosgene and 6mL of anhydrous toluene. After dropping, continue to stir and react for 1 hour under the protection of nitrogen, remove the ice-salt bath, and react at room temperature for 30 minutes, then slowly heat up to reflux. After 4 hours, the reaction solution becomes clear, and 2,4-difluoro-α- Aminoacetophenone hydrochloride 0.38g (2mmol), added anhydrous toluene 10mL, heated to reflux overnight. Cool, filter with suction, wash the filter cake with chloroform 20mL×2, combine the filtrates, wash with saturated NaCl solution, and wash the organic layer with anhydrous NaCl 2 SO 4 After drying, the solvent was recovered under reduced pressure to obtain a tan s...
Embodiment 3
[0031] Embodiment 3: N, the preparation of 3-two (1H-indol-5-yl)-4-(4-chlorophenyl)-4-imidazolidin-2-one-1-formamide (compound IVb2)
[0032] The operation process is the same as in Example 2, except that 2,4-difluoro-α-aminoacetophenone hydrochloride is replaced with 4-chloro-α-aminoacetophenone hydrochloride. A pale yellow solid (compound IVb2) was obtained with a yield of 49.6%. Melting point: 239-241°C.
[0033] 1 H NMR (δ, DMSO-d 6 ): 5.57-5.59 (d, 1H, J = 8.0Hz, indole H), 6.43-6.48 (d, 2H, J = 20Hz, indole H), 6.95-6.97 (d, 1H, J = 8.4Hz, indole H ) 7.15-7.20 (d, 2H, J=8.8Hz, aromatic H), 7.29-7.31 (d, 2H, J=8.8Hz, aromatic H), 7.37-7.39 (d, 2H, J=6.0Hz, indole H ), 7.41-7.45 (m, 3H, indole H×2, imidazolin-2-one H), 7.55 (s, 1H, indole H), 7.83 (s, 1H, indole H), 10.77 (s, 1H, - O=C-N-H-), 11.14(s, 1H, indole N-H), 11.37(s, 1H, indole N-H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com