Peptide for treatment of vascellum generation and uses thereof
一种血管、生成性的技术,应用在小肽及其应用领域,能够解决活性低、弱免疫原性、合成不方便等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
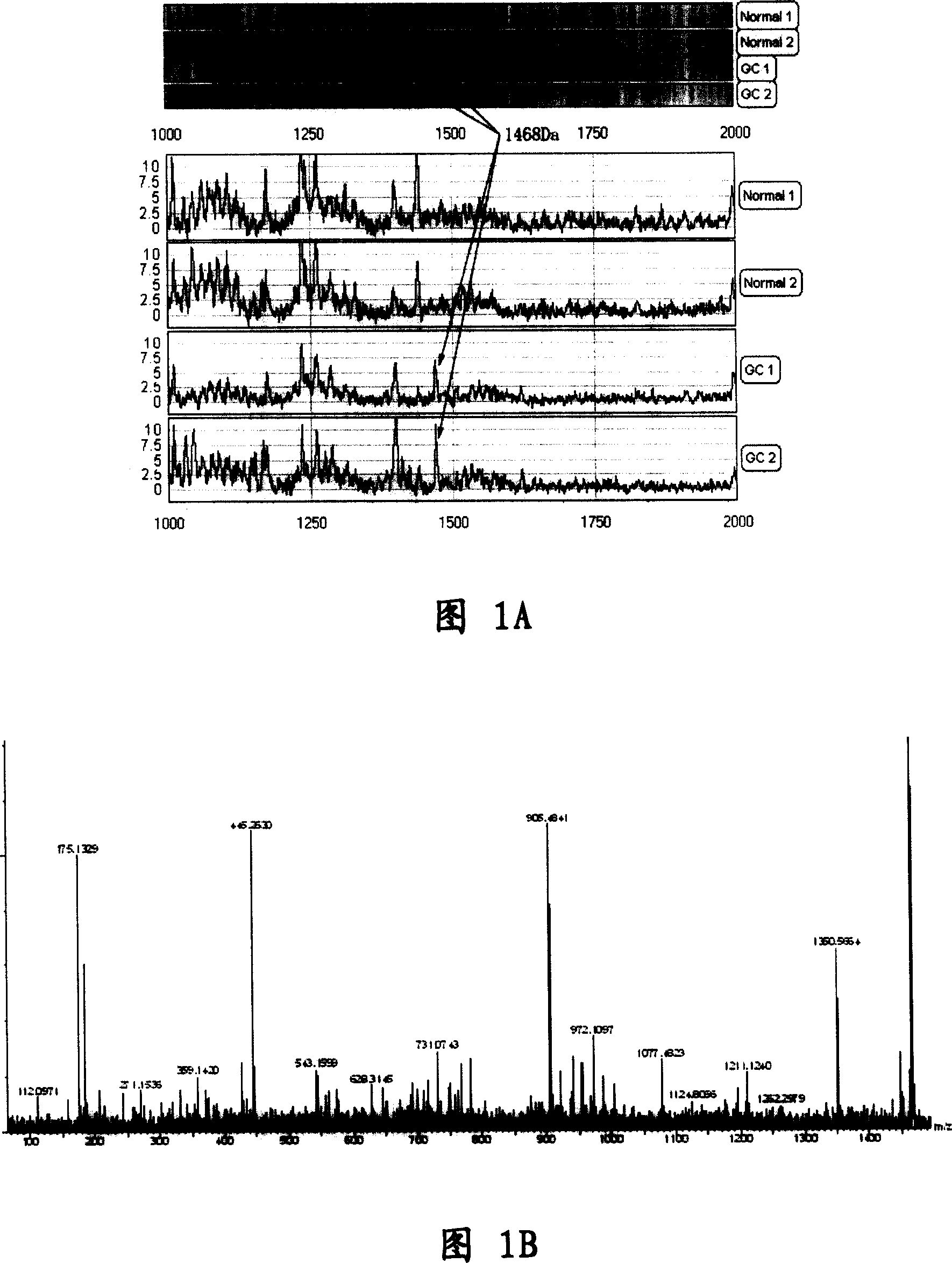
[0065] The determination of embodiment 1.FpAT sequence
[0066] Using Surface-Enhanced Laser Desorption / Ionization-Time-of-Flight Mass Spectrometry (SELDI-TOF-MS) technology, gastric cancer patients (127 cases) and paired normal subjects (100 cases ) serum was analyzed to obtain serum protein expression mass spectrograms of gastric cancer and non-gastric cancer. Through model analysis based on artificial neural network, three low-molecular-weight differential proteins that can effectively distinguish gastric cancer and normal serum were screened out. When the differential protein is used as a group of gastric cancer-specific markers to diagnose gastric cancer, the sensitivity and specificity in the model group were 95.6% and 92.0%, and the accuracy rate was 93.7%; in the blind screening group, the sensitivity and specificity were 85.3% and 88%, the accuracy rate is 86.4%. Among them, a low-molecular-weight differential protein is specifically present in the serum of patients ...
Embodiment 2
[0071] Example 2. Chemical synthesis of FpAT small peptides and truncated small peptides (see Table 2 for each specific sequence)
[0072] FpAT small peptide (H 2 Chemical synthesis of N-Asp-Ser-Gly-Glu-Gly-Asp-Phe-Leu-Ala-Glu-Gly-Gly-Gly-Val-Arg-COOH):
[0073] Put the peptide synthesis column packed with 4-(2',4'-dimethoxyphenyl-Fmoc-aminomethyl)phenoxyacetamidoethyl resin in Pioneer TM peptide synthesizer, and carry out peptide synthesis under nitrogen in the following order:
[0074] 1. Solvate the resin in DMF for 5 minutes;
[0075] 2. Treat the resin with 20% piperidine in DMF for about 15 minutes to remove the protecting group Fmoc on the resin grafted group (or on the resin-bound amino acid α-amino group);
[0076] 3. Wash the resin with DMF for about 1 minute;
[0077] 4. Activate the α-carboxyl group of the first amino acid Arg at the C-terminal with a 0.2M solution of HBTU and HOBT in DMSO-NMP (N-methylpyrrolidone) and a 0.4M solution of diisopropylethylamine i...
Embodiment 3
[0094] Example 3. Homology Analysis of FpAT and VEGF
[0095] Using the analysis program (http: / / www.ebi.ac.uk / Tools / Sequence Analysis-ClustalW), the homology between FpAT and VEGF was analyzed according to the default parameters. The analysis results are as follows:
[0096] FpAT -----DSG--------------EG-------------DFL------------8
[0097] RRGAEESGPPHSPSRRGSASRAGPGRASETMNFLLSWVHWSLALLLYLHH 200
[0098] :** * :**
[0099] VEGF -----AEGGG-----------VR--------------------------15
[0100] AKWSQAAPMAEGGGQNHHEVVKFMDVYQRSYCHPIETLVDIFQEYPDEIE 250
[0101] ***** *:
[0102] It can be seen that the six amino acids Leu-Ala-Glu-Gly-Gly-Gly and at least the five amino acids Ala-Glu-Gly-Gly-Gly are important sequences of the small peptide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com