Rnai therapeutic for respiratory virus infection
A respiratory and viral technology, applied in respiratory diseases, DNA/RNA fragments, antiviral agents, etc., can solve the problems of antigenic drift, limited vaccine potency, and no better treatment plan for influenza virus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0141] siRNA preparation
[0142] In one embodiment, siRNA is synthesized in vivo or in vitro. The cell's endogenous RNA polymerase can mediate in vivo transcription, or the cloned RNA polymerase can be used for in vivo or in vitro transcription. For transcription from transgenes or expression constructs in vivo, regulatory regions (such as promoters, enhancers, silencers, or splice donors and acceptors) can be used to transcribe siRNA. Through specific transcription in organs, tissues, or cell types; stimulation by environmental conditions (such as infection, stress, temperature, ski inducer); and / or engineered transcription at the developmental stage or age can be targeted for inhibition. By introducing recombinant constructs into fertilized eggs, embryonic stem cells, or other pluripotent cells from suitable organisms, transgenic organisms expressing siRNA from the recombinant constructs can be produced.
[0143] In addition, not only siRNA can be used to produce multiple RNA...
Embodiment 1
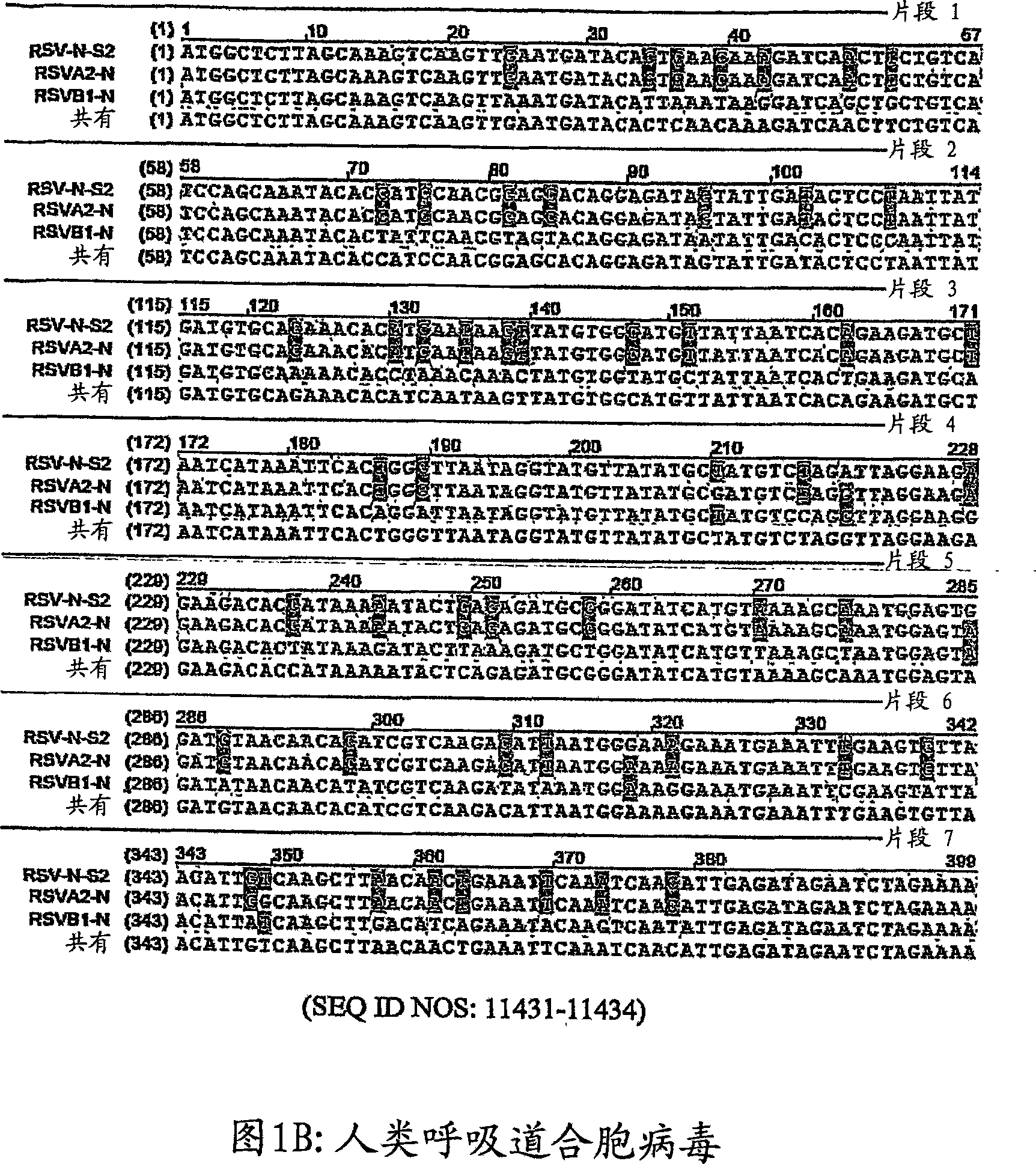
[0198] Example 1: Identification of viral nucleoprotein
[0199] A highly conserved site is considered to be a site or sequence present in most available human influenza sequences. The variant is identified as a 19-mer sequence in a human influenza isolate, similar to the conservative 19-mer sequence, but with only one or a few nucleotide changes. This is important because using siRNA duplexes, RISC (RNA-induced silencing complex) can still initiate RNAi activity. The guide strand (antisense strand) of the siRNA duplex is largely complementary to the target mRNA sequence, but relative to Exact complementation has one or a few nucleotide changes.
[0200] There are eight different RNA segments that make up the influenza virus genome. All analyses are performed separately for each virus fragment. Therefore, for example, only the sequence obtained from the fragment #1 is used to study the conserved sites of the virus fragment #1.
[0201] Obtain the influenza A virus sequence of each...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com