Synergistic modulation of FLT3 kinase using aminopyrimidines kinase modulators and a farnesyl transferase inhibitor
A technology of farnesyltransferase and kinase inhibitor, which is applied in the field of synergistic regulation of FLT3 kinase with aminopyrimidine kinase modulator and farnesyltransferase inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
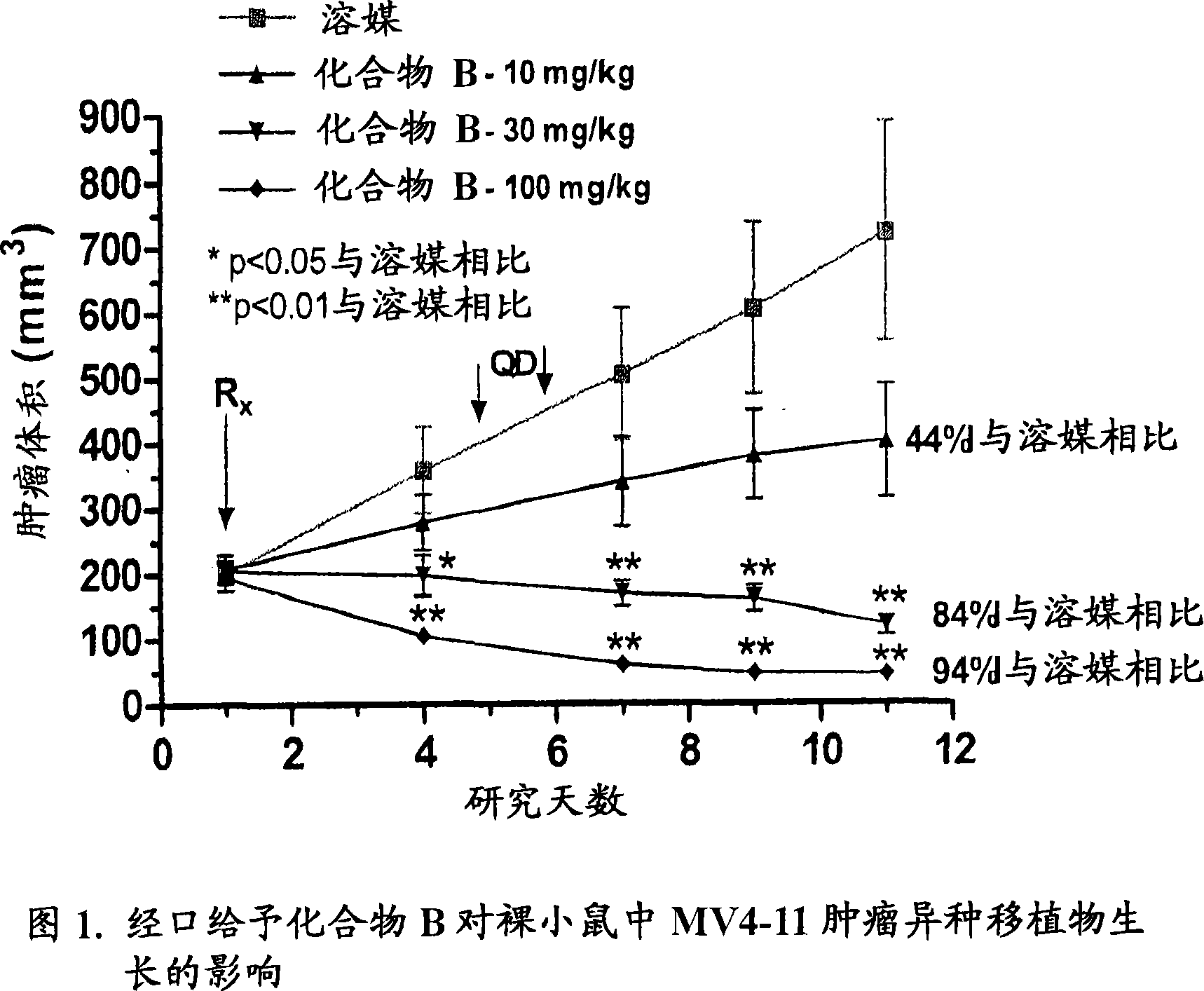
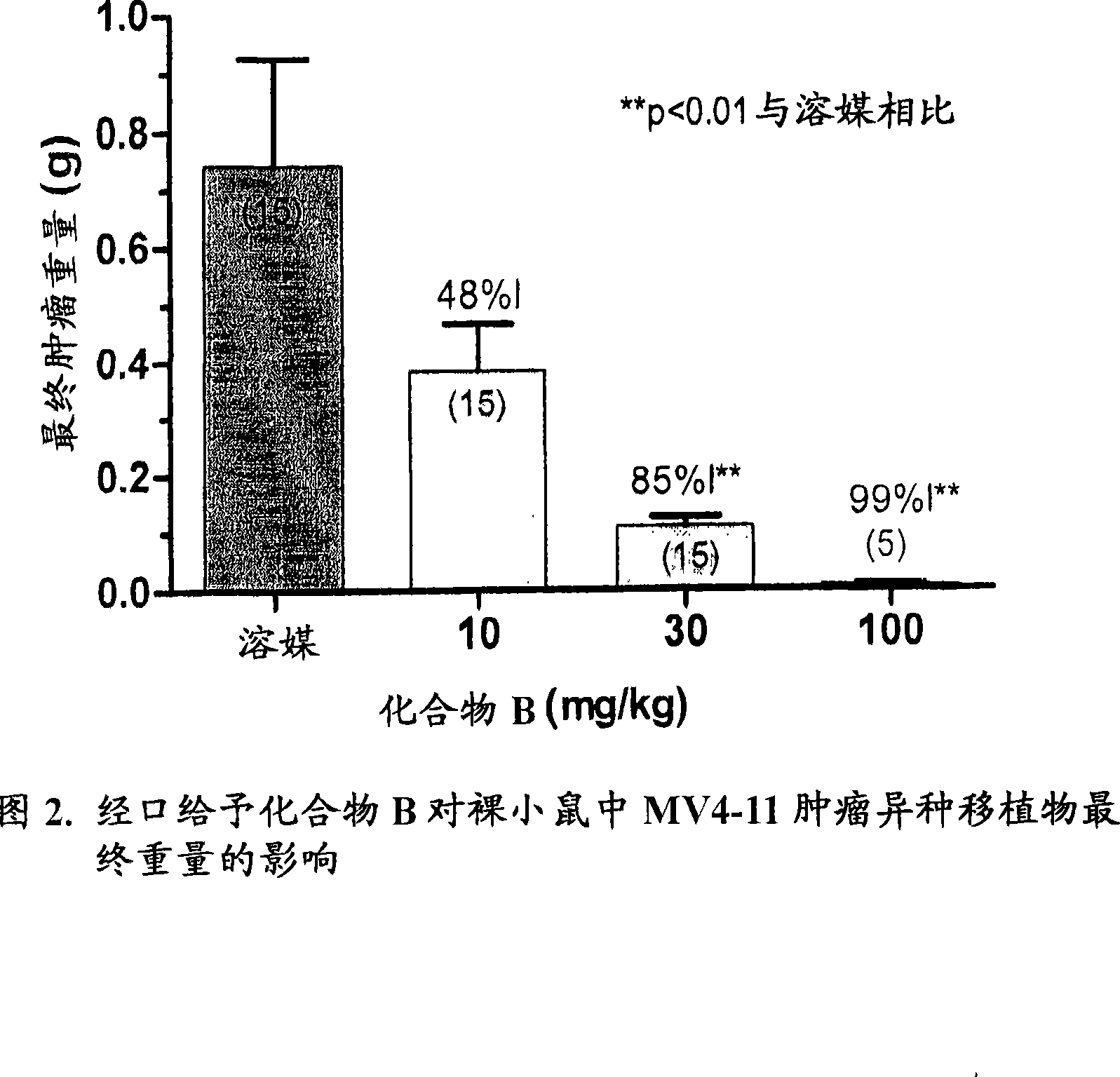
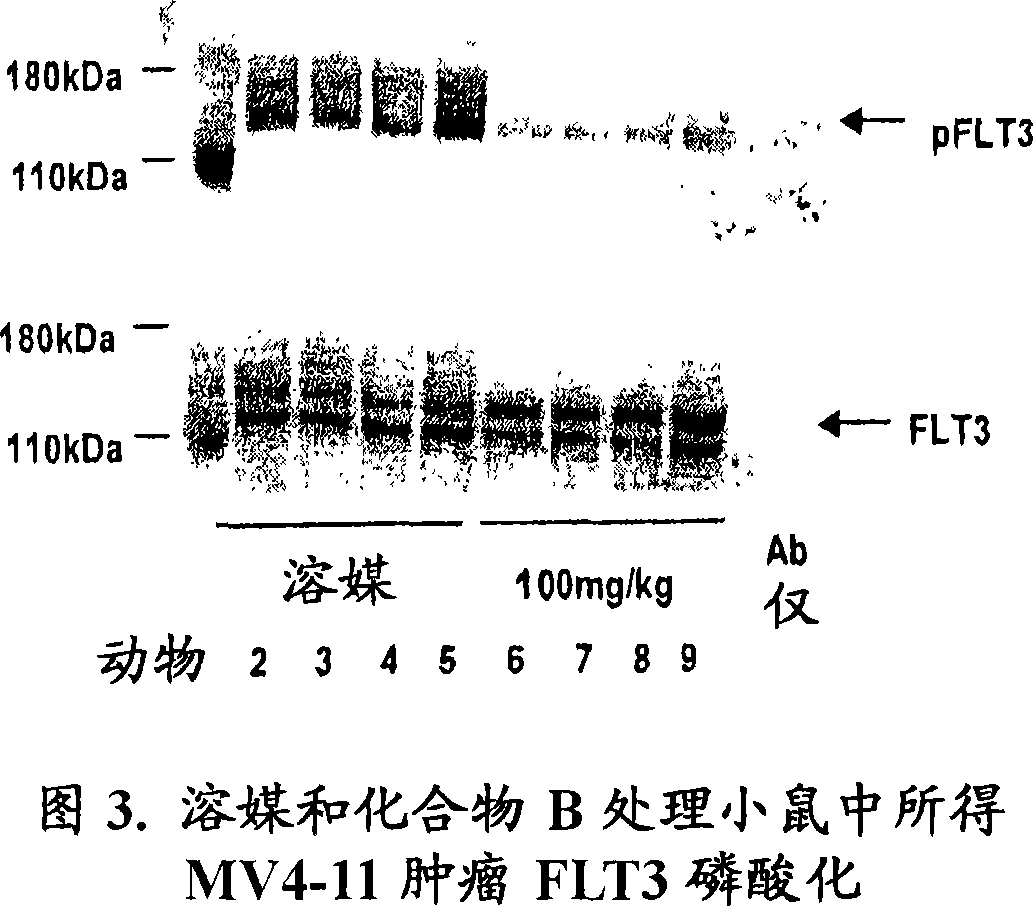
Image
Examples
Embodiment 1
[0627] N-(4-isopropoxy-phenyl)-4-[6-amino-5-(methoxyimino-methyl)-pyrimidin-4-yl]-piperazine-1-carboxamide
[0628]
[0629] a. 4,6-dichloro-pyrimidine-5-carbaldehyde
[0630]
[0631] At 0 °C, DMF (3.2 mL) and POCl 3 (10 mL) was stirred for 1 h, treated with 4,6-dihydroxypyrimidine (2.5 g, 22.3 mmol) and stirred at room temperature for 0.5 h. The heterogeneous mixture was heated to reflux for 3h and the volatiles were removed under reduced pressure. The residue was poured into ice water and extracted 6 times with ether. The organic phase was washed with NaHCO 3 Washed with aqueous solution, Na 2 SO 4 Drying and concentration gave a yellow solid (3.7 g, 95%).
[0632] 1 H NMR (CDCl 3 )δ10.46(s, 1H), 8.90(s, 1H),
[0633] b. 4-Amino-6-chloro-pyrimidine-5-carbaldehyde
[0634]
[0635] Ammonia was bubbled through a solution of 4,6-dichloro-pyrimidine-5-carbaldehyde (1 g, 5.68 mmol) in toluene (100 mL) for 10 min, and the solution was stirred at room temperatur...
Embodiment 2
[0680] N-(4-isopropoxy-phenyl)-4-{6-amino-5-[(2-morpholin-4-yl-ethoxyimino)-methyl]-pyrimidin-4-yl }-piperazine-1-carboxamide
[0681]
[0682] a. 4-Amino-6-piperazin-1-yl-pyrimidine-5-carbaldehyde trifluoroacetate
[0683]
[0684] 4-(6-Amino-5-formyl-pyrimidin-4-yl)-piperazine-1-carboxylic acid tert-butyl ester (235 mg, 0.76 mmol) was dissolved in 50% TFA / CH 2 Cl 2 (10 mL) and the mixture was stirred overnight. Evaporation under reduced pressure afforded a white solid as a pure product which was directly used in the next reaction.
[0685] 1 H NMR (CD 3 OD) δ9.83(s, 1H), 8.29(s, 1H), 4.22(t, J=5.23Hz, 4H), 3.42(t, J=5.42Hz, 4H);
[0686] LC / MS (ESI) C 9 h 14 N 5 O(MH) + The theoretical value is 208.1, and the measured value is 208.1.
[0687] b. N-(4-isopropoxy-phenyl)-4-(6-amino-5-formyl-pyrimidin-4-yl)-piperazine-1-carboxamide
[0688]
[0689] To 4-amino-6-piperazin-1-yl-pyrimidine-5-carbaldehyde trifluoroacetate (0.76mmol) and (4-isopropoxy-phenyl)-ca...
Embodiment 3
[0708] N-(4-isopropoxy-phenyl)-4-{6-amino-5-[(3-hydroxy-propoxyimino)-methyl]-pyrimidin-4-yl}-piperazine- 1-formamide
[0709]
[0710] a. Diphenyl-methanone O-(3-hydroxy-propyl)-oxime
[0711]
[0712] Prepared according to the synthesis method of Example 2c.
[0713] 1 H NMR (CDCl 3 )δ7.30-7.52(m, 10H), 4.35(t, J=5.83Hz, 2H), 3.73(t, J=5.85Hz, 2H), 1.95(m, 2H).
[0714] b. 3-aminooxy-propan-1-ol hydrochloride
[0715]
[0716] Prepared according to the synthesis method of Example 2d.
[0717] 1 H NMR (CD 3 OD) δ4.26(t, J=6.75Hz, 2H), 3.66(t, J=6.11Hz, 2H), 2.51(m, 2H).
[0718] c.N-(4-isopropoxy-phenyl)-4-{6-amino-5-[(3-hydroxy-propoxyimino)-methyl]-pyrimidin-4-yl}-piperazine- 1-formamide
[0719]
[0720] Prepared as described in Example 2e except substituting 3-aminooxy-propan-1-ol for O-(2-morpholin-4-yl-ethyl)-hydroxylamine.
[0721] 1 H NMR (CD 3 OD) δ8.22(s, 1H), 8.08(s, 1H), 7.21(d, J=8.95Hz, 2H), 6.83(d, J=9.01Hz, 2H), 4.52(m, 1H), 4.28 (t, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com