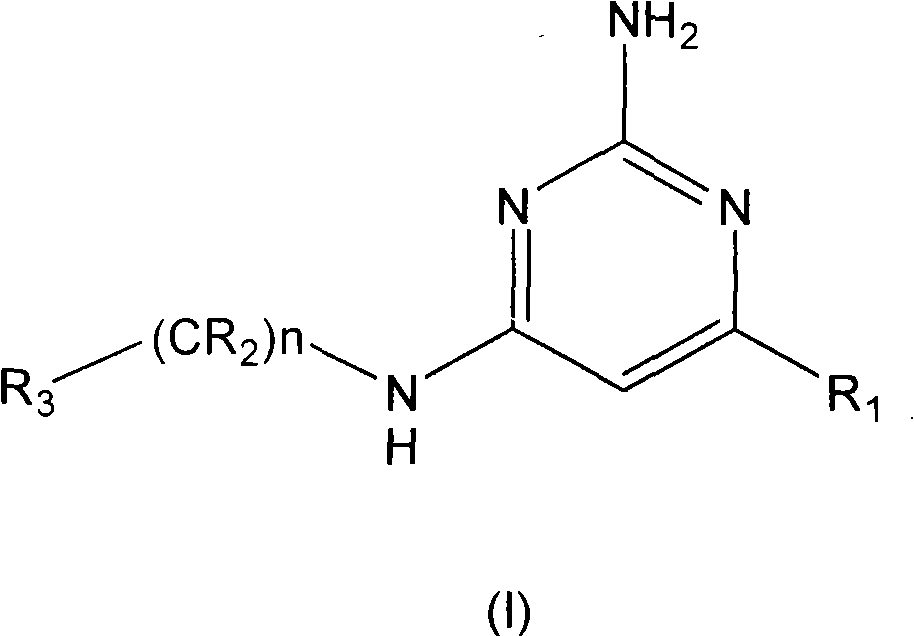
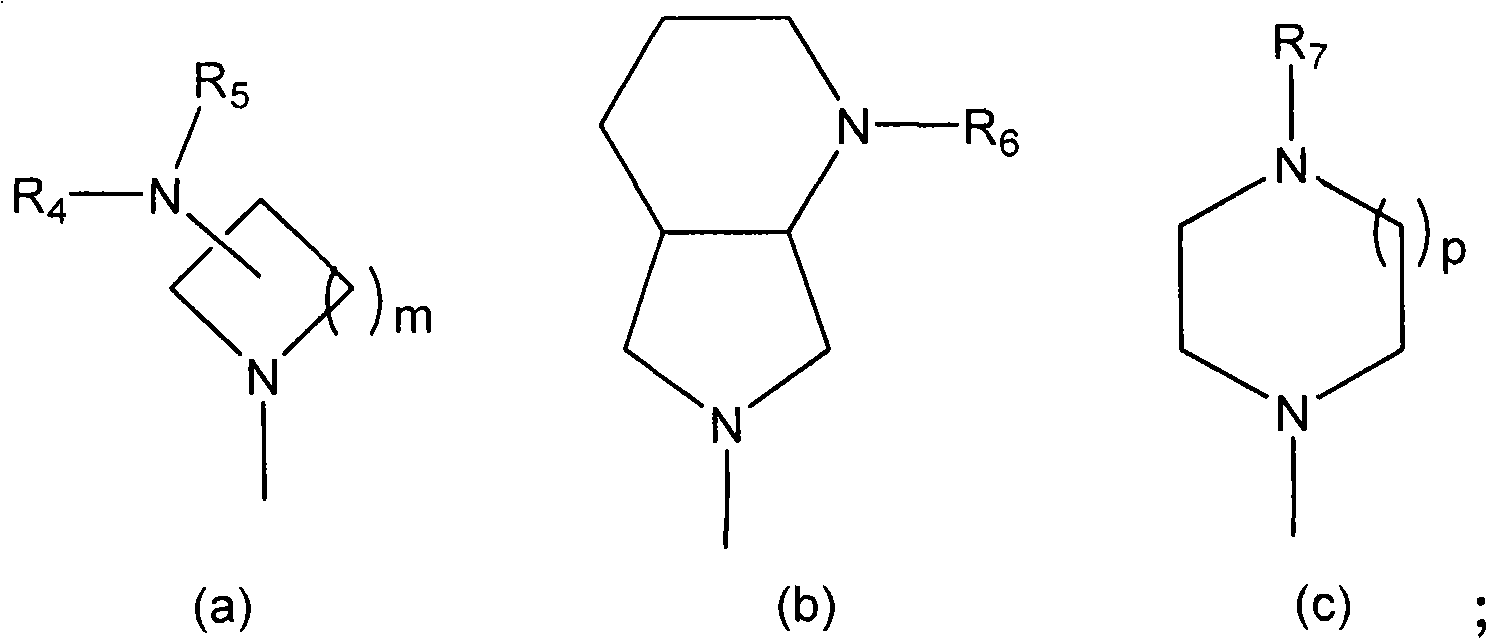
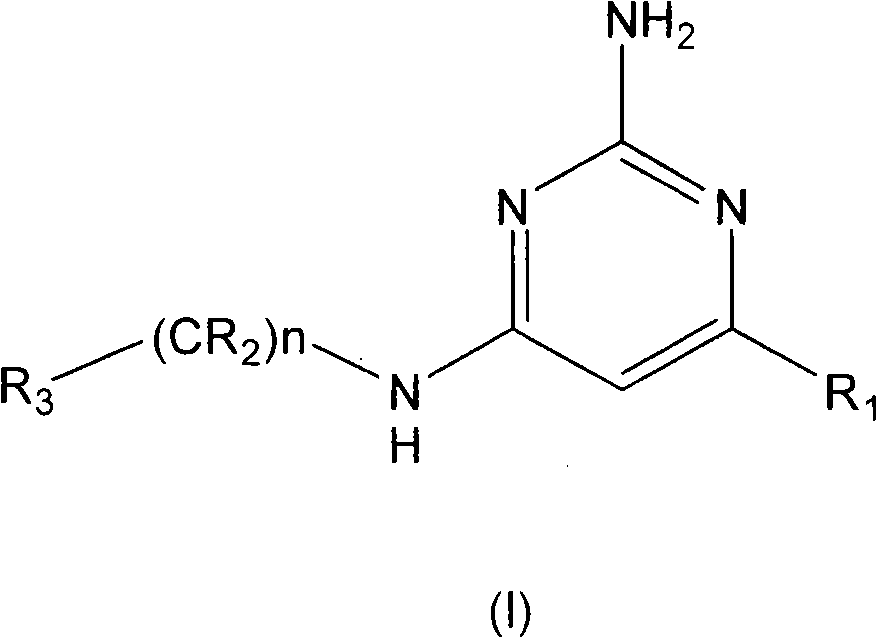
2-aminopyrimidine derivatives as modulators of the histamine h4 receptor activity
An alkyl and phenyl technology, which can be used in drug combinations, respiratory diseases, skin diseases, etc., and can solve problems such as low homology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 5
[0217] Tert-Butylmethyl[(3R)-pyrrolidin-3-yl]carbamate
[0218] (a) tert-Butyl [(3R)-1-benzylpyrrolidin-3-yl] methyl carbamate
[0219] To (3R)-1-benzyl-N-methylpyrrolidin-3-amine (10g, 52.55mmol) cooled at 0°C in 115mL CH 2 Cl 2 Add 15mL CH of di-tert-butyl dicarbonate (11.6g, 53.07mmol) to the solution 2 Cl 2 Solution. The resulting solution was stirred at room temperature for 18 hours. The solvent was evaporated, and the crude product was chromatographed on silica gel using a hexane / AcOEt mixture of increasing polarity as an eluent to obtain 14.5 g of the title compound (yield: 95%).
[0220] LC-MS (Method 1): t R =9.55min; m / z=291(MH + ).
[0221] (b) Title compound
[0222] A solution of the compound (14.5 g, 50.14 mmol), Pd / C (10%, 50% water) (3 g) and ammonium formate (12.7 g, 200.5 mmol) obtained as above in a mixture of MeOH (390 mL) and water (45 mL) Heat under reflux for 5 hours. The reaction was filtered through celite, and the filtrate was washed with AcOEt and MeOH. ...
example 6
[0225] Tert-Butylazetidin-3-yl (methyl) carbamate
[0226] (a) tert-Butyl[1-(diphenylmethyl)azetidin-3-yl]methyl carbamate
[0227] Follow the procedure similar to that described in section 5a of the reference example, but use 1-(diphenylmethyl)-N-methylazetidine-3-amine instead of (3R)-1-benzyl-N-methyl Pyrrolidine-3-amine to obtain the desired compound with a yield of 73%.
[0228] LC-MS (Method 1): t R =10.14min; m / z=353(MH + ).
[0229] (b) Title compound
[0230] A solution of the compound (6.18 g, 17.53 mmol) obtained above in 60 mL MeOH and 15 mL AcOEt was purged with argon. Pd / C (10%, 50% water) (929mg) was added, then the solution was purged again with argon, and the 2 Stir under the atmosphere for 18 hours. The reaction was filtered through celite, and the filtrate was washed with AcOEt and MeOH. The solvent was evaporated to dryness to obtain a mixture of 5.66 g of the title compound and one equivalent of diphenylmethane, which was used directly.
[0231] 1 H NMR (300MH...
example 7
[0233] Tert-Butylazetidin-3-yl (ethyl) carbamate
[0234] (a) tert-Butyl [1-(diphenylmethyl)azetidin-3-yl] carbamate
[0235] Follow the similar process as described in section 5a of the reference example, but use 1-(diphenylmethyl)azetidine-3-amine instead of (3R)-1-benzyl-N-methylpyrrolidine-3 -Amine to obtain the title compound with a yield of 61%.
[0236] LC-MS (Method 1): t R =9.07min; m / z=339(MH + ).
[0237] (b) tert-butyl [1-(diphenylmethyl)azetidin-3-yl] ethyl carbamate to 55% NaH (985 mg, 22.5 mmol), THF cooled at 0°C (40 mL) and EtI (2.34 mL, 28.7 mmol) were added to the compound (6.9 g, 20.5 mmol) obtained above, and the resulting mixture was stirred at room temperature for 18 h. Then additional 55% NaH (500 mg, 11.45 mmol) and EtI (1.3 mL, 16.2 mmol) were added, and the mixture was stirred at room temperature for 18 h. A few drops of water are added and the mixture is partitioned between AcOEt and water. Pass the organic phase through Na 2 SO 4 Dry and concentrate to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com