Anti-glypican-3 antibody
A phosphatidylinositol and proteoglycan technology, applied in the direction of antibodies, anti-animal/human immunoglobulins, immunoglobulins, etc., can solve problems such as functions that have not been well elucidated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
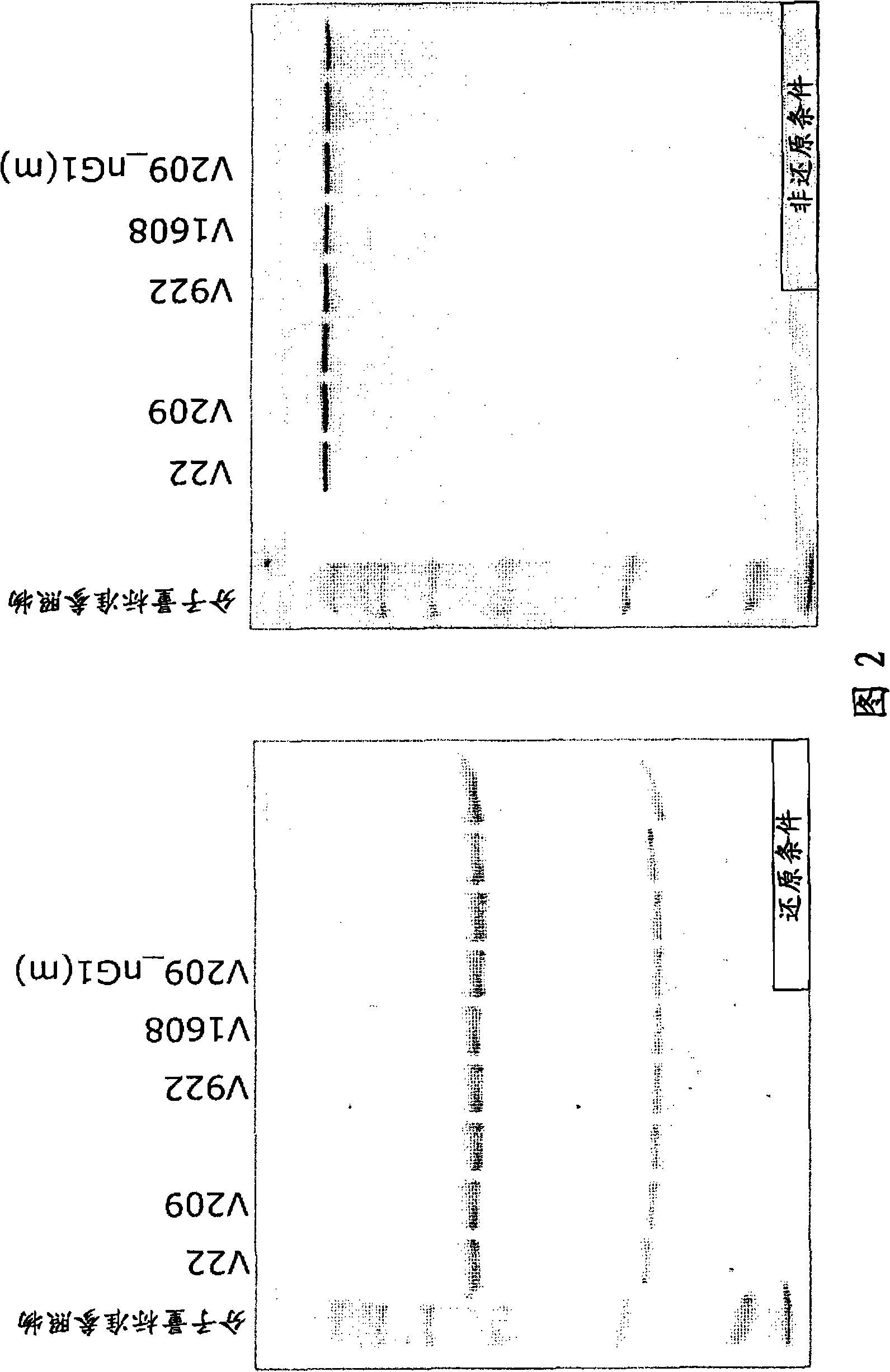
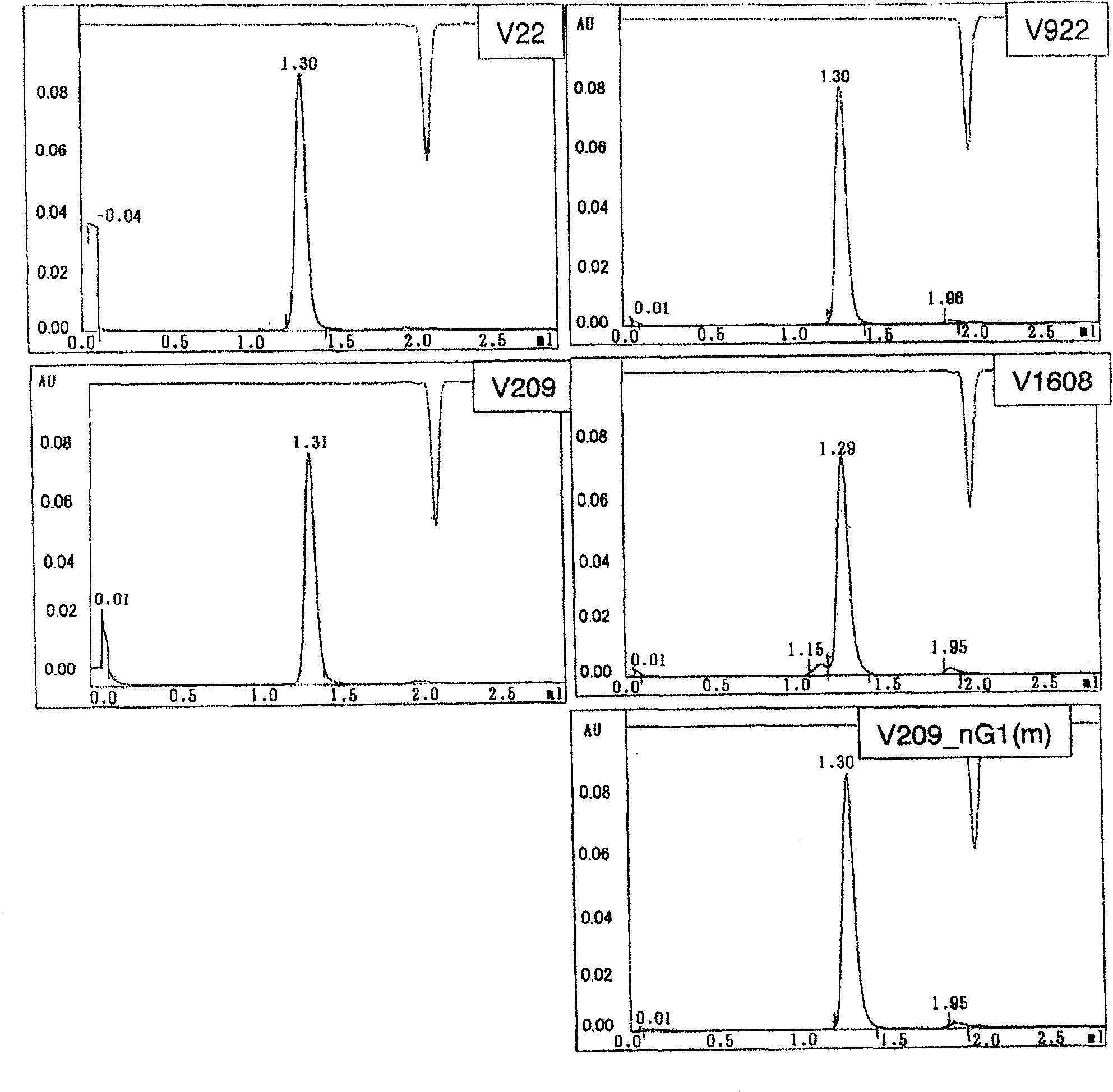
[0112] Example 1: Generation of Fc-modified anti-GPC3 antibodies:
Embodiment 1-1
[0113] Example 1-1: Preparation of Fc box for mutagenesis:
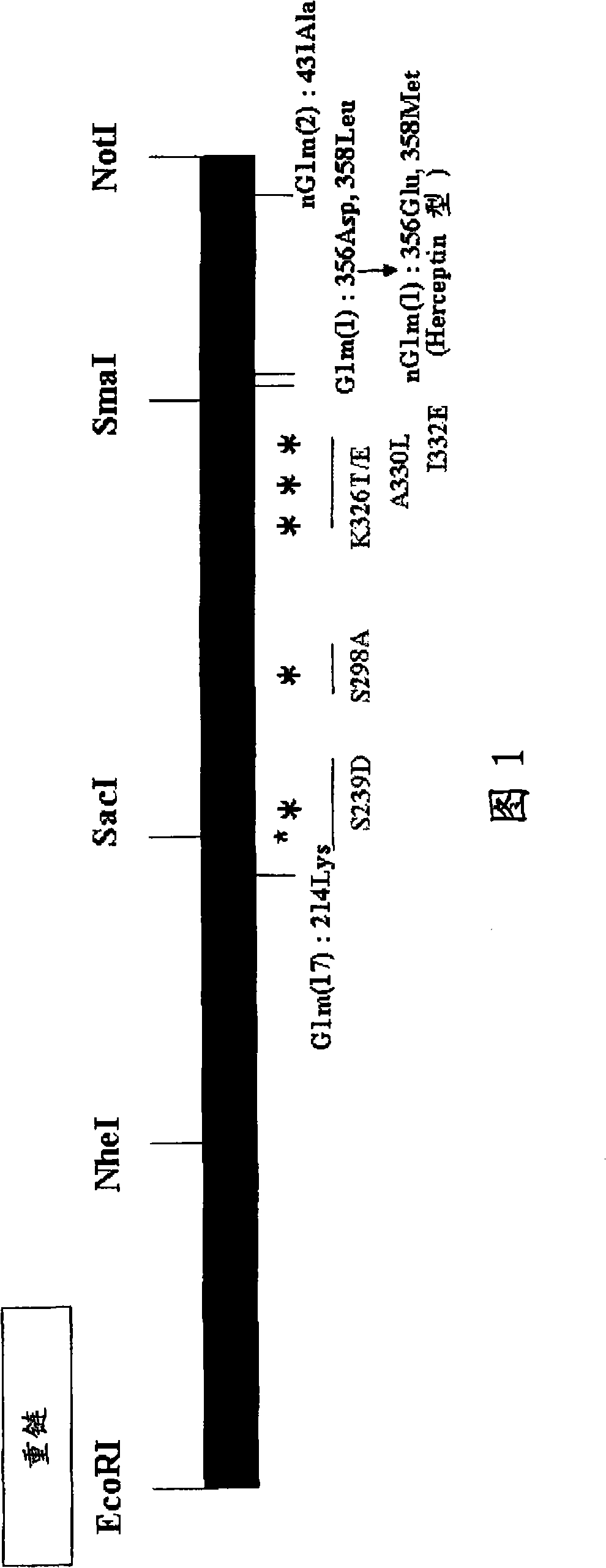
[0114] The Fc-modified humanized anti-GPC3 antibody has the amino acid substitutions shown in the table below in the H-chain amino acid sequence shown in SEQ ID NO:19. Figure 1 shows the structure and preparation strategy of the Fc-modified antibody of the present invention.
[0115] V22
I332E
V209
S239D / A330L / I332E
V212
S239D / S298A / I332E
V922
S239D / K326T / I332E
V1608
S239D / S298A / K326T / I332E
V209nG1m(1)
S239D / A330L / I332E / D356E / L358M
[0116] Using the primers shown in SEQ ID NO: 1 to NO: 9, Fc-mutation cassettes for the construction of five Fc-modified antibodies named V22, V209, V212, V922 and V1608 were generated according to the PCR walking method. Specifically, universal primers F1 and universal R1 are used for V22, V209 and V922; primers 212-F1 and 212-F1 are used for V212 and V1608. Perform the first-stage PCR in the following...
Embodiment 1-2
[0143] Example 1-2: Preparation of vectors expressing Fc-modified anti-GPC3 antibodies
[0144] According to the gene (H-chain, SEQ ID NO: 10; L-chain, SEQ ID NO: 11) encoding the humanized anti-glypican-3 antibody previously prepared by the inventors, a A vector for expressing the Fc-modified anti-GPC3 antibody of the present invention, which is referred to as "wild type" in the following examples.
[0145] The amino acid sequences of the H-chain variable region and the L-chain variable region of the wild-type humanized anti-GPC3 antibody are shown in SEQ ID NO: 21 (ver.k) and SEQ ID NO: 22 (ver.a), respectively middle. The CDR sequences of the wild-type humanized anti-GPC3 antibody are shown below.
[0146] H-chain
[0147] CDR1 DYEMH (SEQ ID NO: 23)
[0148] CDR2 ALDPKTGDTAYSQKFKG (SEQ ID NO: 24)
[0149] CDR3 FYSYTY (SEQ ID NO: 25)
[0150] L-chain
[0151] CDR1 RSSQSLVHSNGNTYLH (SEQ ID NO: 26)
[0152] CDR2 KVSNRFS (SEQ ID NO: 27)
[0153] CDR3 SQNTHVPPT (SEQ ID ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com