Oxcarbazepine pharmaceutical formulation and its method of preparation, wherein oxcarbazepine has a broad and multi-modal particle size distribution
A kind of oxcarbazepine particle, the technology of oxcarbazepine, it is applied in the preparation containing oxcarbazepine and preparation of this pharmaceutical preparation, the field of pharmaceutical preparation containing carbamazepine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1: Preparation of a 10:90 ratio multimodal oxcarbazepine formulation
[0111] In the first step 1, a starting dispersion formulation is prepared from large oxcarbazepine. 1200 g of large oxcarbazepine raw material (RM) [d(0.9)=248.5] was dispersed in 4800 g of purified aqueous solution of 240 g of hypromellose (Pharmacoat 603). Add the Pharmacoat to the water and mix until you get a clear mixture. Subsequently, large oxcarbazepine was added to the Pharmacoat water mixture and dispersed with a rotor-stator mixer (Brogtec) for about 30 min. Measure the particle size distribution of large oxcarbazepine by laser diffraction (S type Malvern laser diffraction granulometer (Malvern Mastersizer)), the results are as follows:
[0112] Oxcarbazepine
d(0.1)
d(0.5)
d(0.9)
big oxcarbazepine
25.1
86.1
308.1
[0113] In the second step 2, the particle size of large oxcarbazepine was reduced by high pressure homoge...
Embodiment 2
[0125] Example 2: Preparation of a 1:5 ratio multimodal oxcarbazepine formulation
[0126] Initial four steps are with embodiment 1. In the final step 5 of preparing the granule mixture and tablets, the sprayed granules and the granules of step 4 were mixed together in a ratio of 16.6:83.3, respectively. In preparing the 600 mg dose, a mixture of the two granules is prepared, wherein the nebulized granules contain 500 mg of active material and the mixed granules contain 100 mg of active material. Therefore, the final formulation including a lubricant as an additional excipient is as follows:
[0127] Formulation of Example 2 (Batch #4)
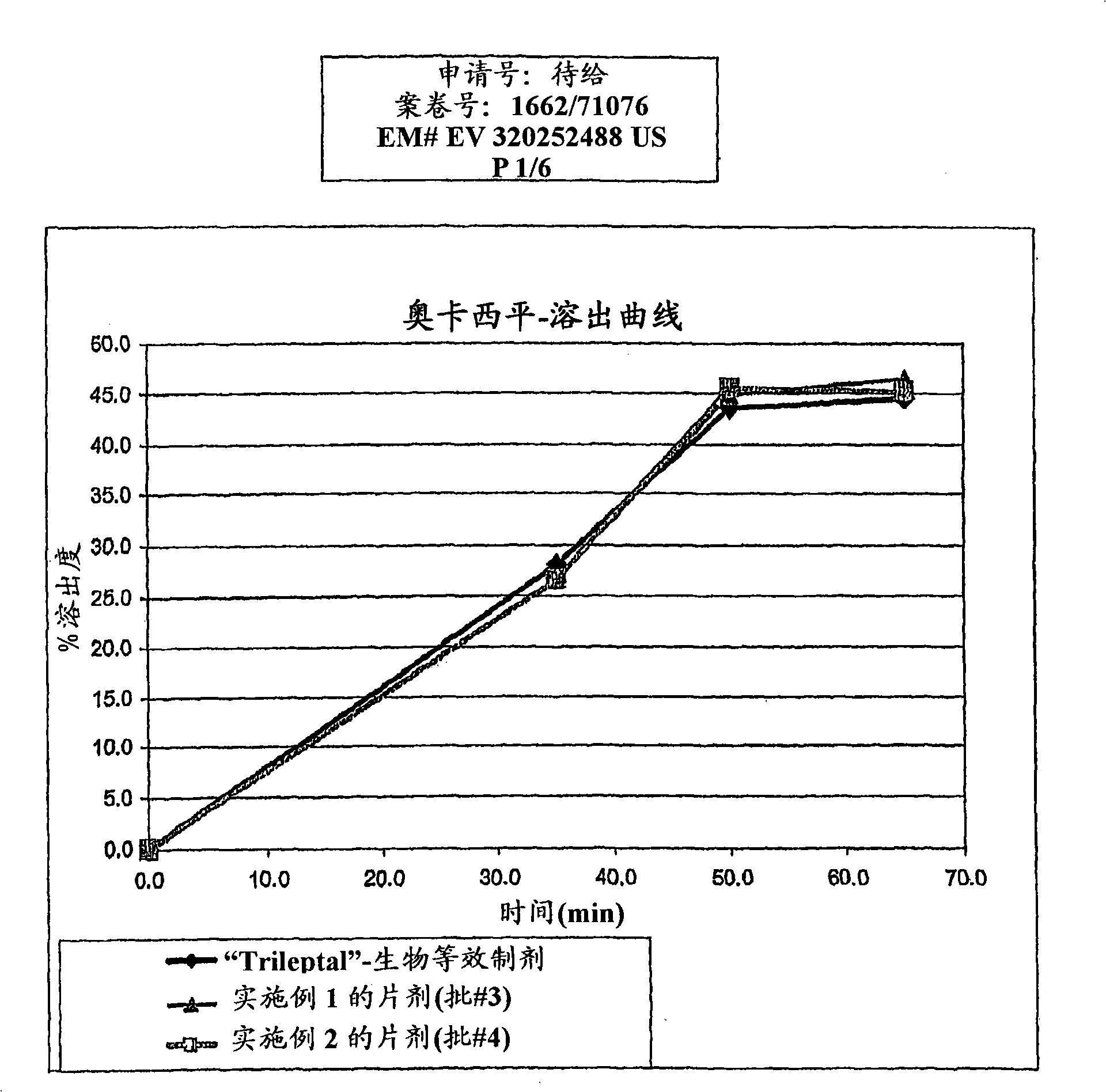
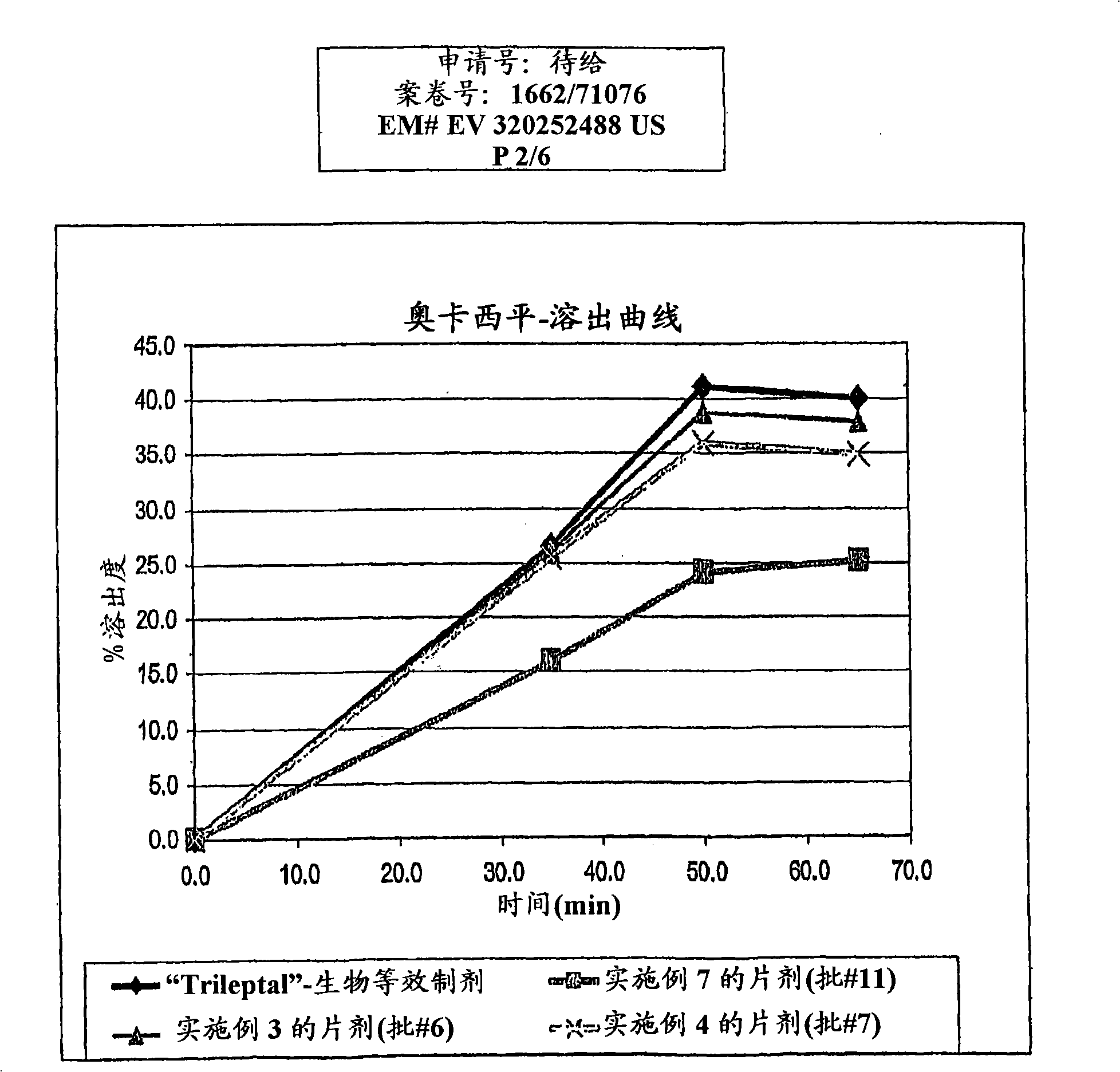
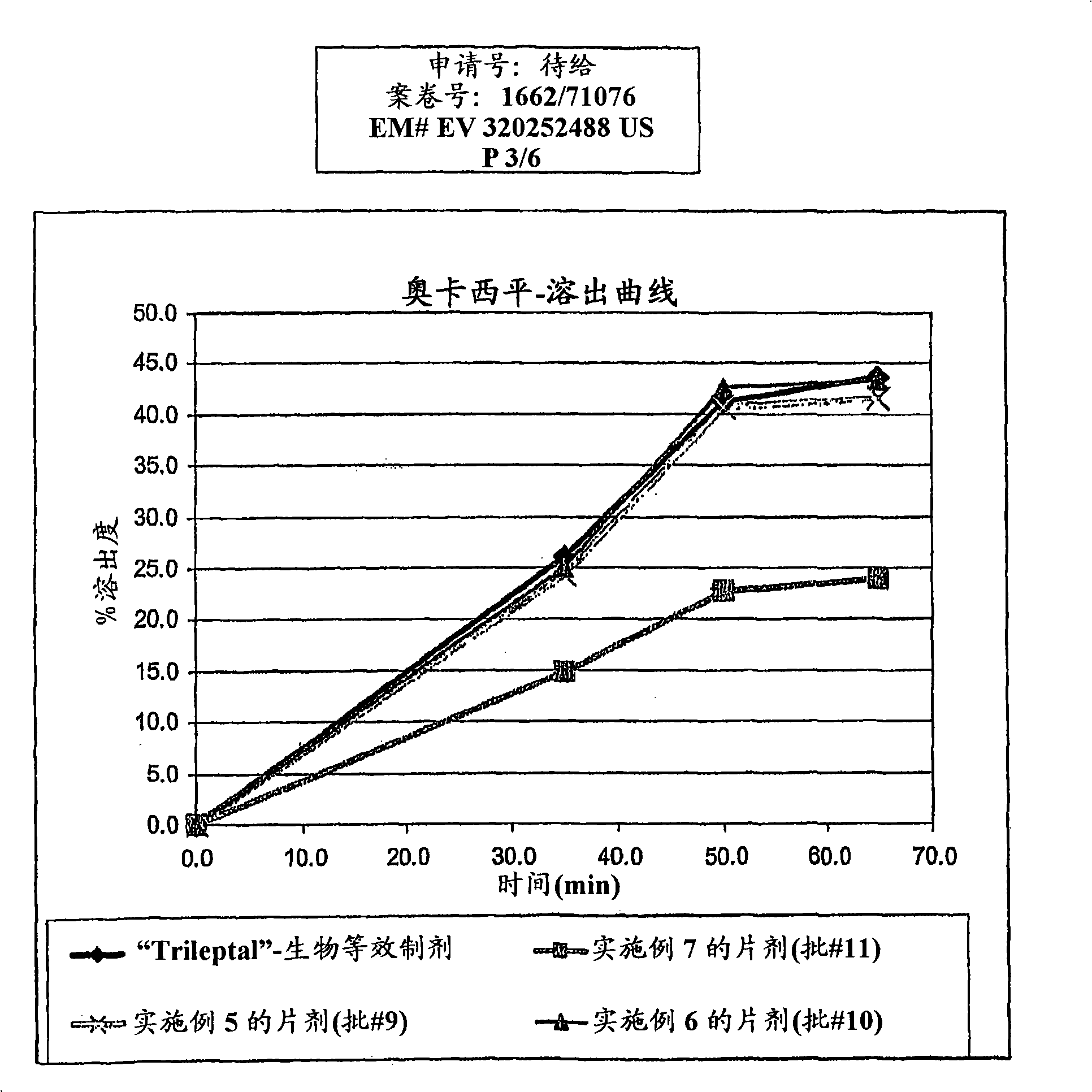
[0128] The granule mixture was then compressed into tablets and subjected to dissolution testing. The observed dissolution rates were similar to Dissolution rates of bioequivalent formulations, such as figure 1 shown in . The particle size of oxcarbazepine used in this example was estimated to be: d(0.5) was 0.7 microns, wit...
Embodiment 3
[0129] Example 3: Using a combination of sprayed granules and mixed granules, a 55:45 ratio was prepared multimodal oxcarbazepine formulation
[0130] Initial three steps are with embodiment 1. Oxcarbazepine granules [d(0.5)=30.5] were prepared by wet granulation in step 4. The preparation of large oxcarbazepine granules is as follows:
[0131]
[0132]
[0133] Measure the particle size distribution of the large oxcarbazepine RM used for granulation by laser diffraction (S type Malvern laser diffraction particle size analyzer (MalvernMastersizer)), the results are as follows:
[0134] Oxcarbazepine
[0135] The final step 5 involves the preparation of the granule mixture and tablets. The sprayed granules of step 3 and the mixed granules of step 4 were mixed together separately in a ratio of 45:55. In preparing the 600 mg dose, a mixture of the two granules is prepared, wherein the sprayed granules contain 270 mg of active material and the mixed granules...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com