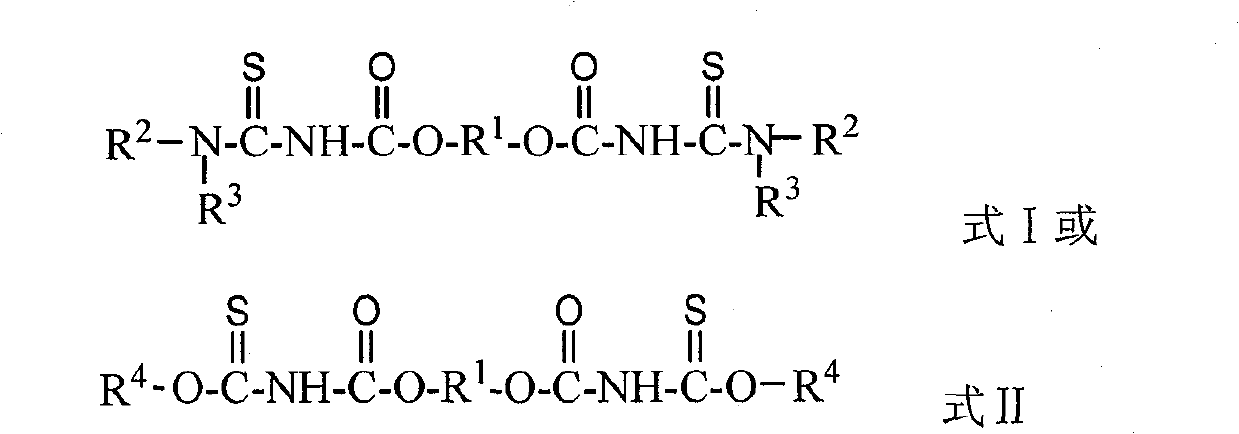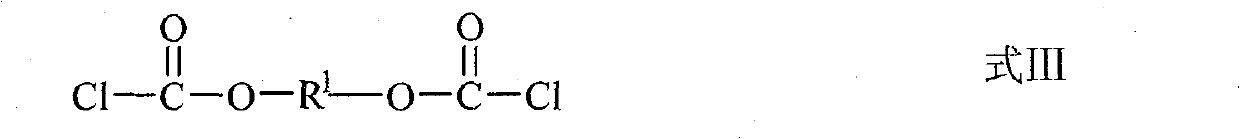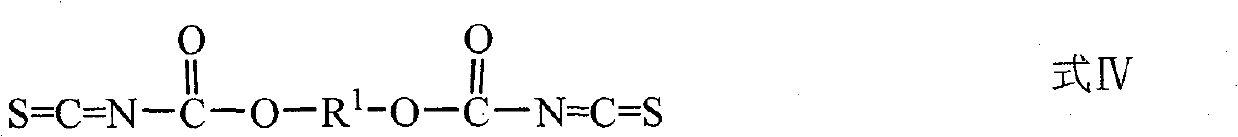Use of diester isosulfocyanate in sulphide ore floation and preparation method thereof
A technology of diester group diisothiocyanate and diester group dithiocarbamate, which is applied in the field of preparation of such compounds, can solve the problems of poor selectivity and low level of comprehensive utilization of sulfide ores, and achieve simple preparation process, Good selectivity, efficient flotation separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 1, the preparation of 4-butylene bis(oxycarbonyl isothiocyanate)
[0041] 38.0 parts NH 4 SCN was dissolved in 70 parts of water, and the mixture was added dropwise to 43.2 parts of butylene bischloroformate under stirring, and 0.24 parts of bis(4-N, N-dimethylaminophenyl) methane was added to control the reaction temperature It is about 0°C to 6°C, and reacts for 3h under stirring. After the stirring was stopped, the mixture was left to stand for about 30 minutes at a temperature of about 2° C., and the mixture was separated into an aqueous phase and an organic phase. After separating and removing the water phase, the obtained orange-red organic phase is the desired 1,4-butylene bis(oxycarbonyl isothiocyanate). The product yield based on ethylene dichloroformate was 93.6%.
Embodiment 2
[0042] Example 2 The preparation of 2,2'-ethylene ether group bis(oxycarbonyl isothiocyanate)
[0043] Add 24.3 parts of NaSCN to 50 parts of dichloromethane, and at the same time add 2.0 parts of PEG-400, and add a solution consisting of 23.1 parts of diethylene glycol dichloroformate and 30 parts of dichloromethane dropwise to the above reaction solution under stirring , control the reaction temperature at about 5°C to 15°C, and react for 4 hours under stirring, and the obtained orange-red dichloromethane solution contains the target product 2,2'-ethylene ether bis(oxycarbonyl isothiocyanate). The yield based on diethylene glycol dichloroformate was 95.2%.
Embodiment 3
[0044] Example 3: Synthesis of O, O'-dibutyl-N, N'-butanediesteryl dithiocarbamate
[0045] Take 0.1 mol of 1,4-butylene bis(oxycarbonyl isothiocyanate) synthesized according to Example 1, add 0.3 mol of n-butanol to it under stirring, and react with stirring at 40° C. for 2 hours after the addition is complete. During the reaction process, the color of the reaction solution gradually turns orange-red to bright yellow, and the reaction solution containing O, O'-dibutyl-N, N'-butanediesteryl dithiocarbamate is obtained, which can be used as sulfide ore without purification. Flotation collectors. Based on 1,4-butylene bis(oxycarbonyl isothiocyanate), the yield of O,O'-dibutyl-N,N'-butanediesteryl dithiocarbamate was 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com