Preparation method of p-aminodiphenylamine
A technology for p-aminodiphenylamine and nitrodiphenylamine, which is applied in the field of preparing p-aminodiphenylamine, can solve the problems of unfavorable large-scale industrial production, low product quality, low product yield and the like, and achieves remarkable economic and social benefits, The effect of high product conversion rate and high number of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
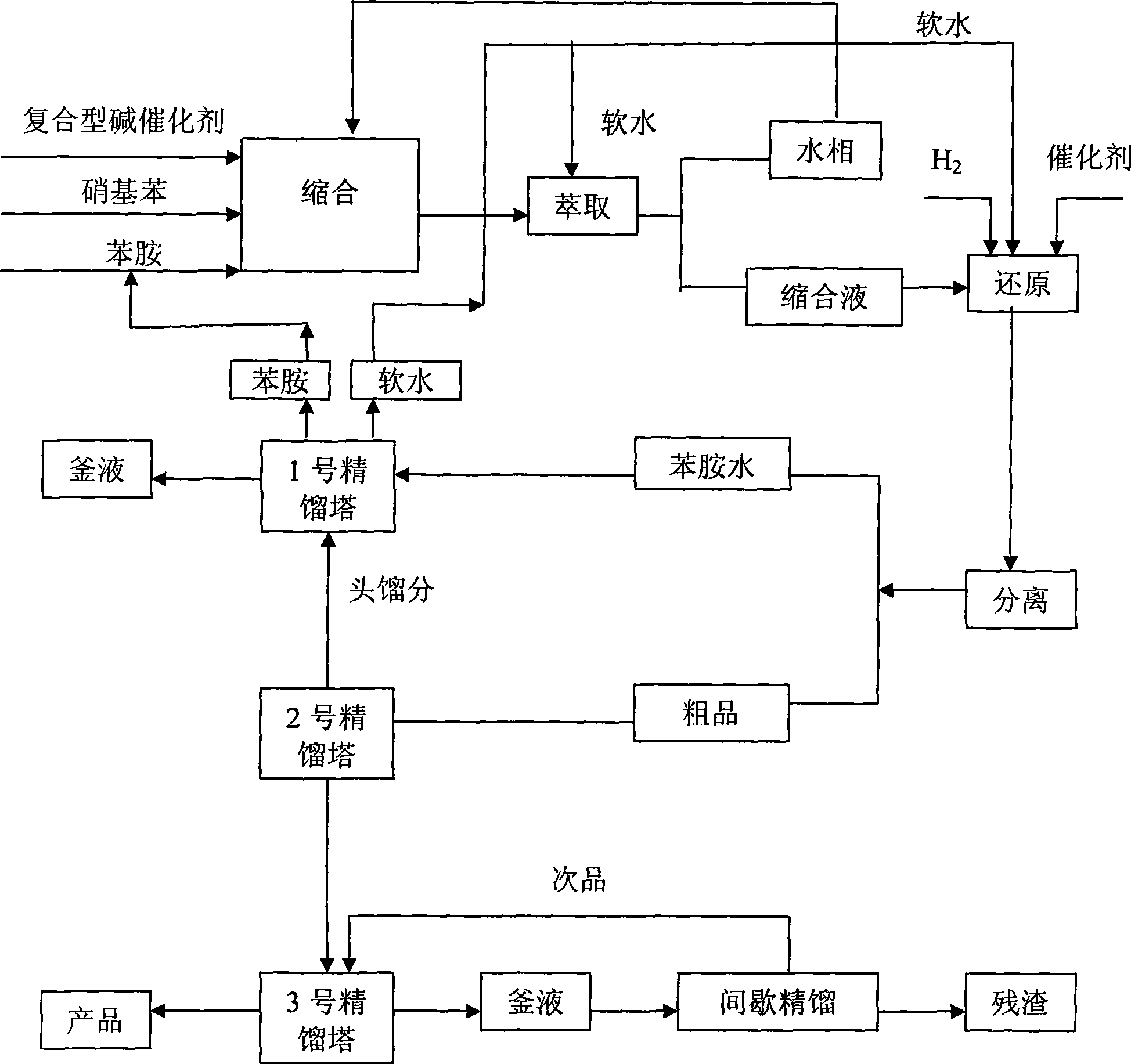
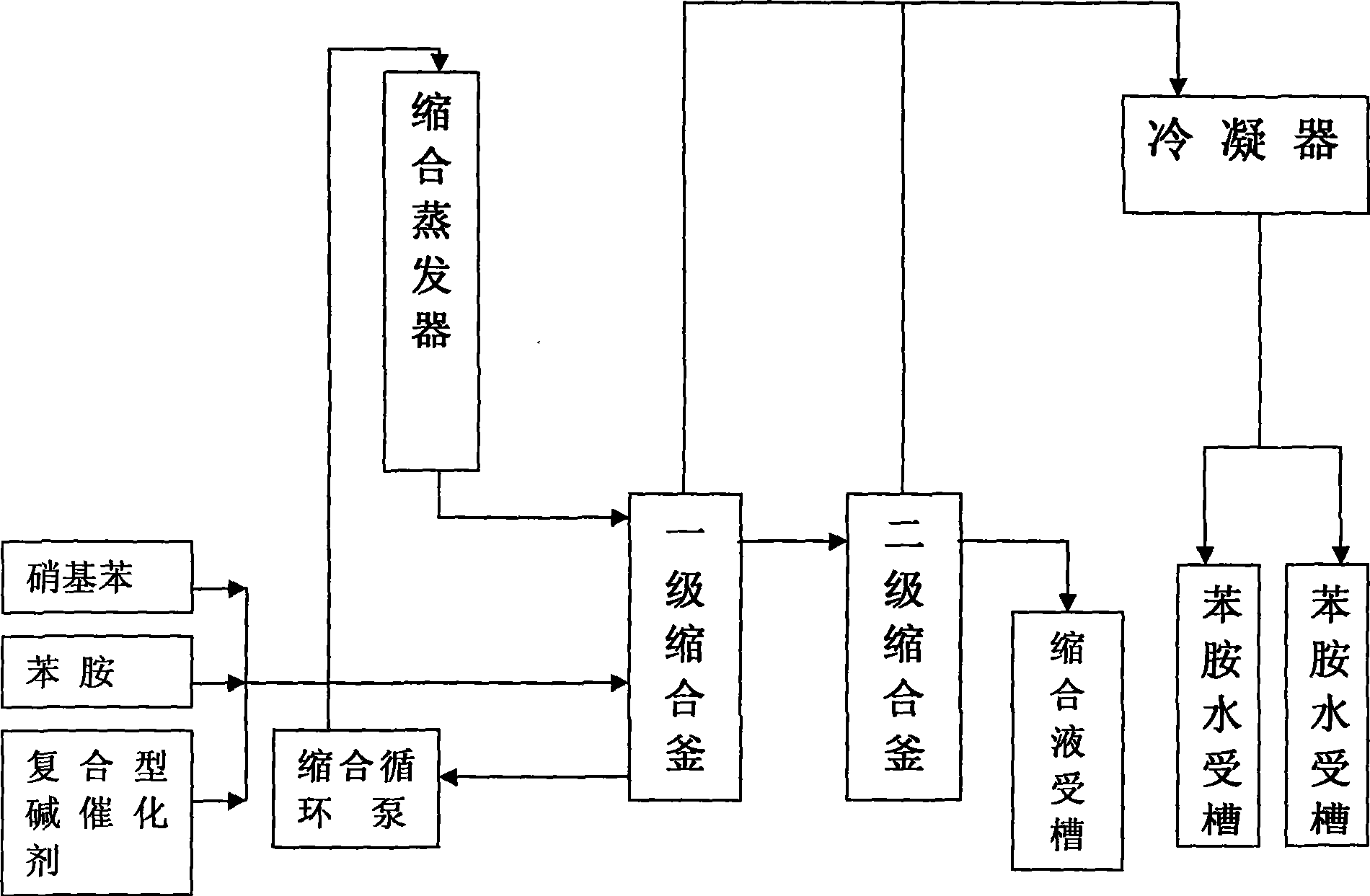
[0033] The preparation method of the composite base catalyst is to add the molar ratio of tetraalkylammonium hydroxide, alkali metal hydroxide, and tetraalkylammonium salt to (3~8):(1~4):(0.5~4) Into a certain amount of water, control the temperature, and stir evenly to reach the composite base catalyst.
[0034] 2. Extraction process:
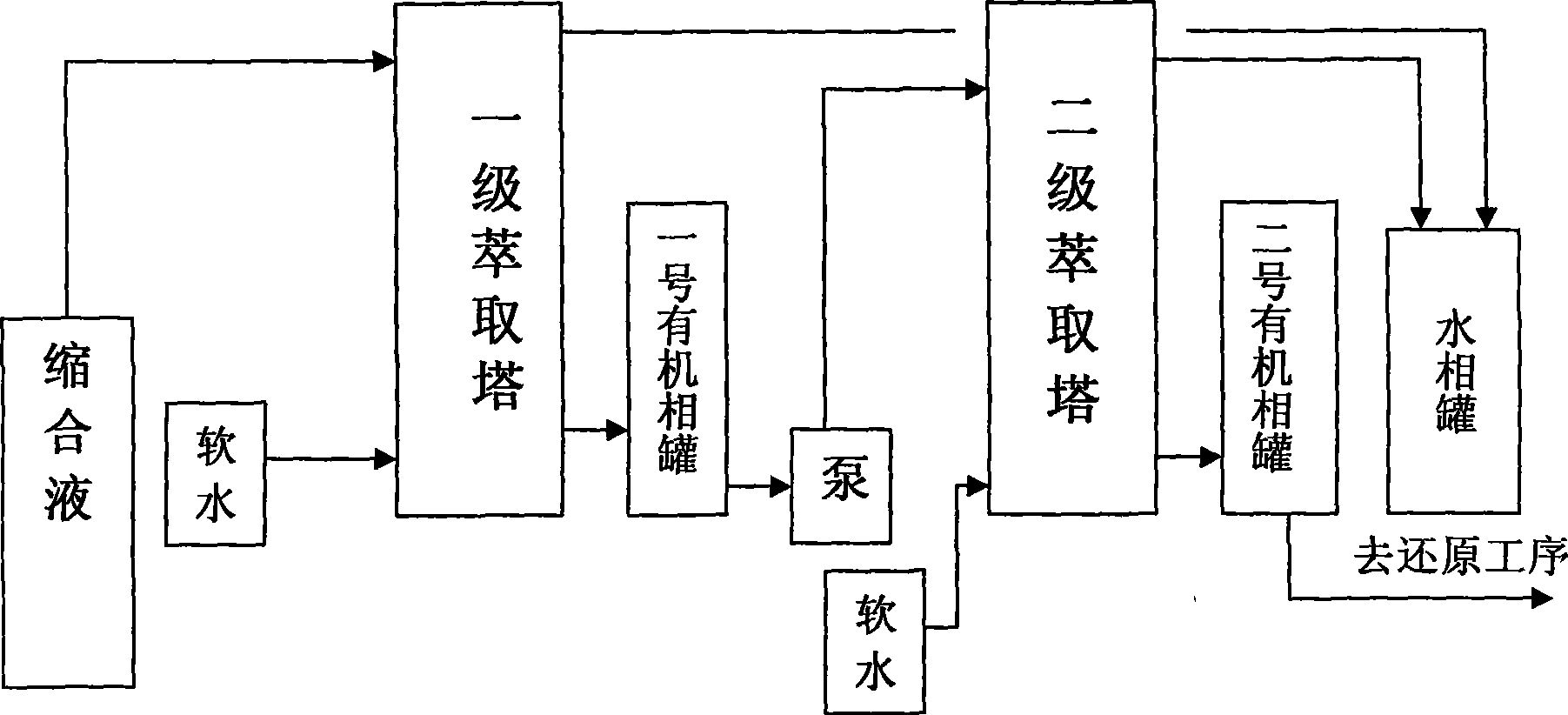
[0035] The main task of the extraction process is to separate the water phase and the organic phase in the condensation liquid. The extraction liquid adopts the soft water from the condensation process, and the ratio of soft water to condensation liquid is 1:2~1:6 (volume), such as 1:2, 1:3, 1:4, 1:5, 1:6, etc., Preferably 1:3~1:5, enter the second or above multi-stage extraction tower for extraction, the extracted water phase re-enters the condensation kettle to participate in the condensation reaction, and the extracted organic phase enters the reduction process with a palladium-carbon catalyst. Hydrogenation reduction reaction.
[0036] The sof...
Embodiment 1
[0065] Example 1: Weigh 30 kg of tetraalkylammonium hydroxide, potassium hydroxide and tetraalkylammonium bicarbonate in a ratio of 3:2:4 by massage, and add 70 kg of soft water and stir until all are melted to form a concentration It is a 30% composite base catalyst.
Embodiment 2
[0066] Example 2: Weigh 20 kilograms of tetraalkylammonium hydroxide, sodium hydroxide and tetraalkylammonium sulfate at a ratio of 5:4:0.5 by a molar ratio, add 180 kilograms of soft water and stir until all are melted, and the concentration is 10% composite base catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com