Ungulates with genetically modified immune systems
A technology for ungulates and animals, applied in the field of ungulates with genetically modified immune systems, which can solve problems such as the unknown sequence and arrangement of immunoglobulin genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
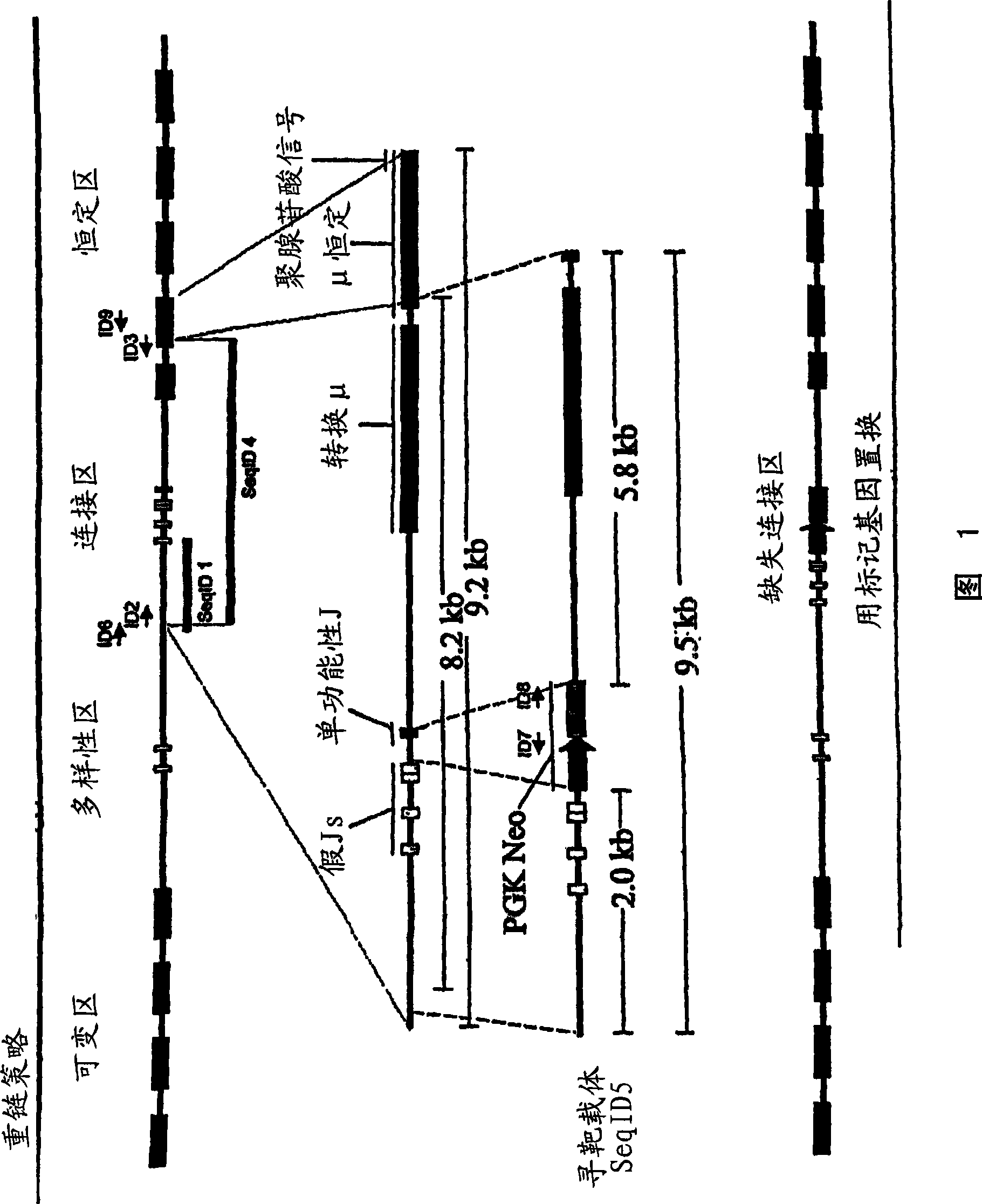
[0374] Example 1: Porcine heavy chain targeting and generation of porcine animals lacking heavy chain expression
[0375] A portion of the porcine Ig heavy chain locus was isolated from a 3X redundant porcine BAC library. Typically, a BAC library can be generated by fragmenting total porcine genomic DNA, which can then be used to obtain a BAC library representing at least three times the genome of the entire animal. BACs containing porcine heavy chain immunoglobulins are then selected by hybridization of probes selective for porcine heavy chain immunoglobulins described herein.
[0376]The sequence from the clone (Seq ID 1) was used to generate a primer complementary to part of the J region (this primer is represented by Seq ID No. 2). Separately, a primer complementary to a part of the mu constant region of the Ig heavy chain (this primer is represented by Seq ID No. 3) was designed. These primers were used to amplify a fragment of the porcine Ig heavy chain (represented by...
Embodiment 2
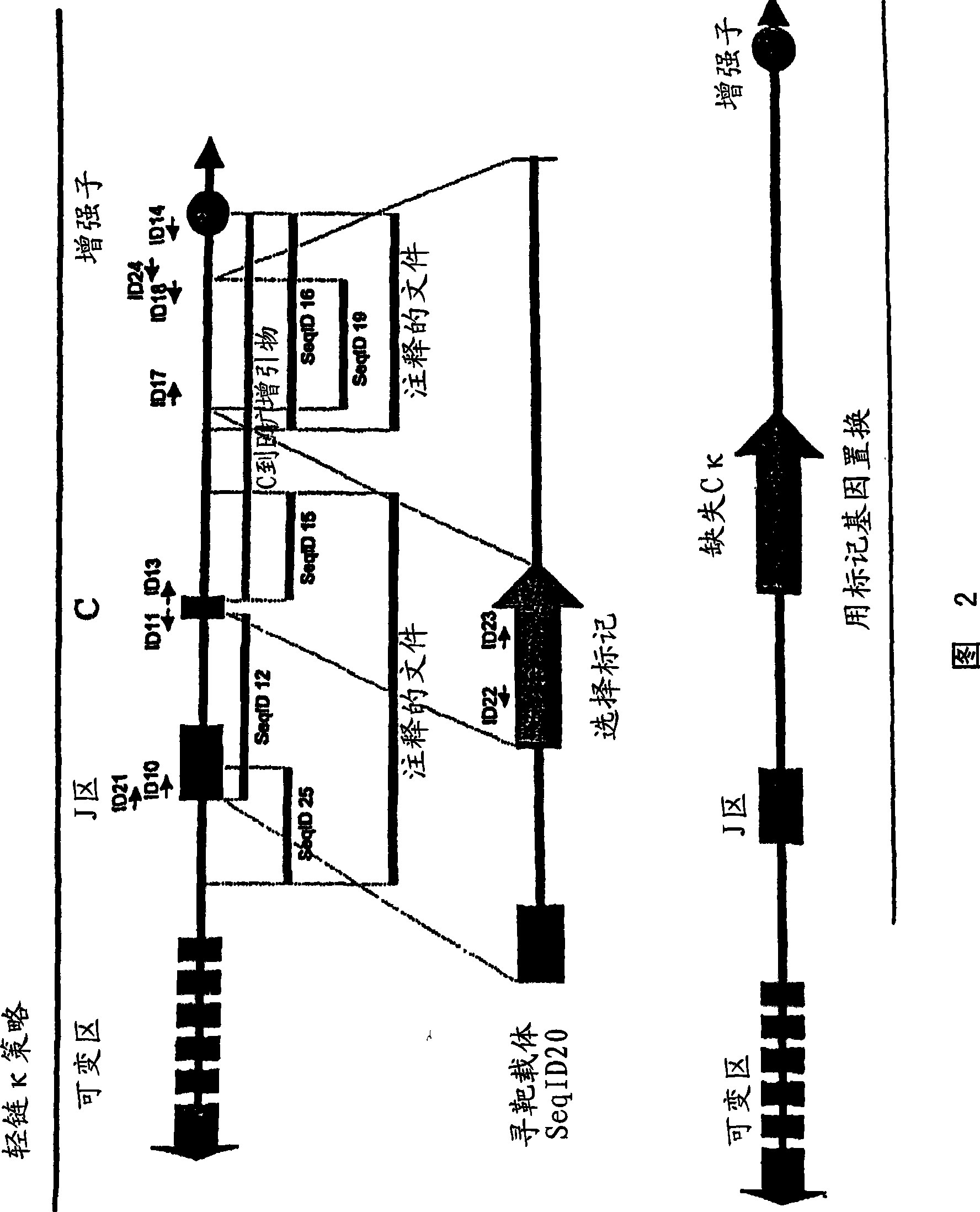
[0403] Example 2: Porcine kappa light chain targeting and generation of pigs lacking kappa light chain expression
[0404] A portion of the porcine Ig kappa chain locus was isolated from a 3X redundant porcine BAC library. Typically, a BAC library can be generated by fragmenting total porcine genomic DNA, which can then be used to obtain a BAC library representing at least three times the genome of the entire animal. BACs containing porcine kappa light chain immunoglobulins can then be selected by hybridization of probes selective for porcine kappa light chain immunoglobulins as described herein.
[0405] A fragment of porcine Ig light chain kappa was amplified using a primer complementary to part of the J region (this primer is represented by Seq ID No. 10) and a primer complementary to part of the kappa C region (this primer was represented by Seq ID No. 11). The resulting amplification primers were cloned into a plasmid vector and maintained in Stable2 cells at 30°C (Seq I...
Embodiment 3
[0425] The characterization of embodiment 3 porcine lambda loci
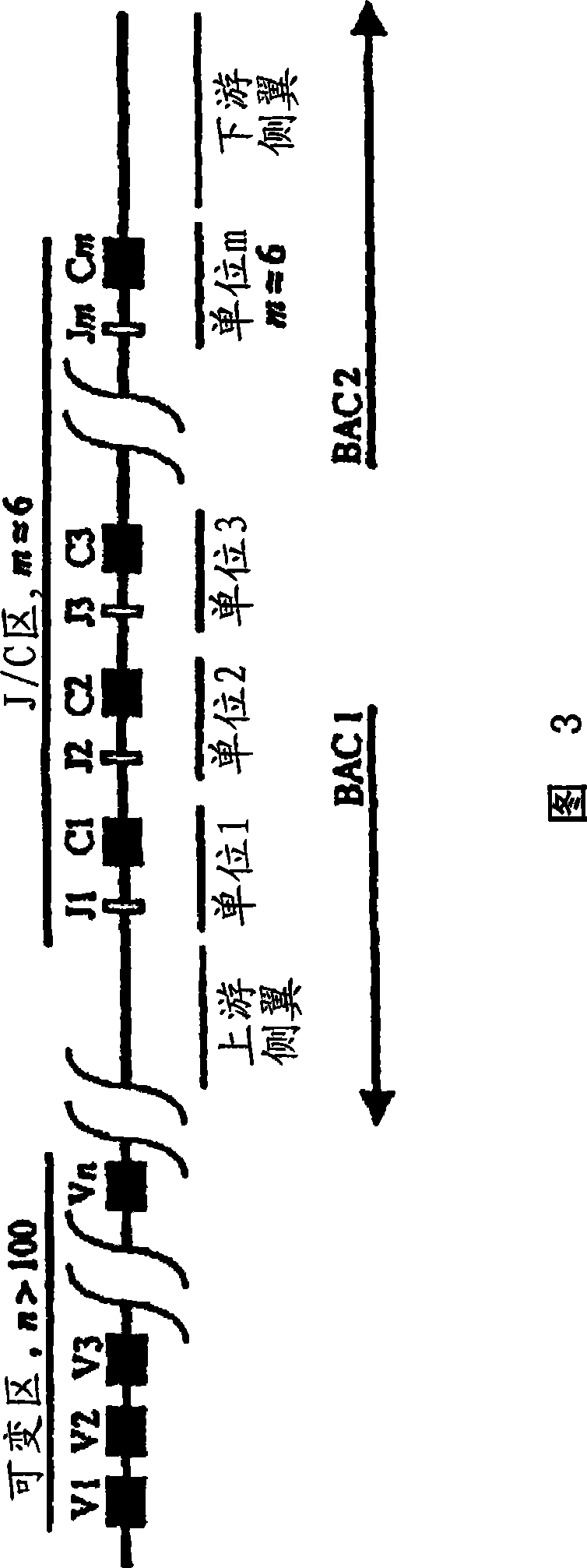
[0426] To disrupt or disable porcine lambda, targeting strategies have been devised that allow removal or disruption of regions of the lambda locus, including the concatemer of J to C expression cassettes. BAC clones containing portions of the porcine genome can be generated. A portion of the porcine Ig lambda chain locus was isolated from a 3X redundant porcine BAC library. Typically, the BAC library can be generated by fragmenting the total genomic DNA of the pig, which is then used to obtain a BAC library representing at least three times the genome of the intact animal. BACs containing porcine lambda chain immunoglobulin can then be selected by hybridization of probes selective for porcine lambda chain immunoglobulin as described herein.
[0427] BAC clones containing the λJ-C flanking regions (see Figure 3) can be independently fragmented and subcloned into plasmid vectors. Individual subclones have been...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com