Cannibinoid receptor modulators
A solvate, pharmaceutical technology for use in the field of cannabinoid receptor modulators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0854] Preparation of bis(hetero)aryl-isoindol-1-ones
[0855] General process A:
[0856]
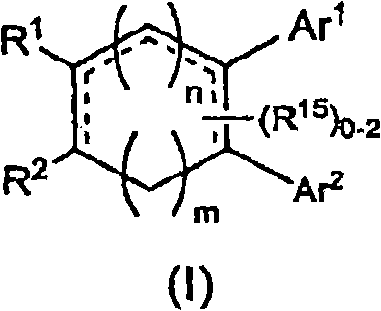
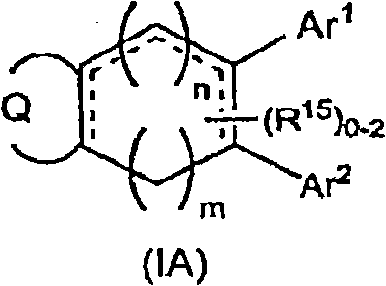
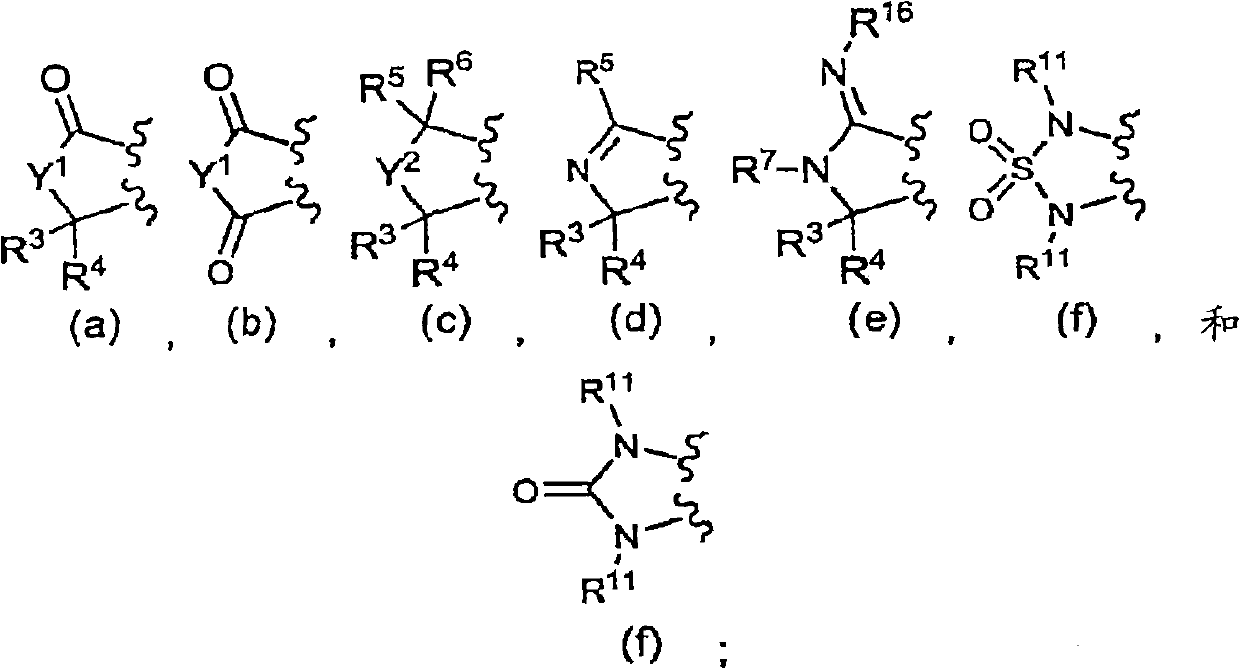
[0857] Diaryl-isoindol-1-ones, diheteroaryl-isoindol-1-ones and aryl-heteroaryl-isoindol-1-ones of formula (I) can be prepared by various methods , for example, condensing an aryl or heteroaryl substituted dienoic acid or acid chloride with an aryl or heteroaryl substituted unsaturated amine to form a trienamide, or making a diaryl, diheteroaryl or aryl -Condensation of a heteroaryl-substituted dienoic acid or acid chloride with an unsaturated amine followed by cyclization of the resulting trienamide by an intramolecular Diels-Alder reaction to yield a di(hetero)aryl-tetrahydro-isoindole- 1-Kones, which can be further modified as desired (eg, by reduction or alkylation, etc.).
[0858] Aldehyde a can be reacted with crotyl phosphonate to provide ester b which, after saponification, can be reacted with oxalyl chloride to give acid chloride c. Sonagashira coupling of aryl or hetero...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com