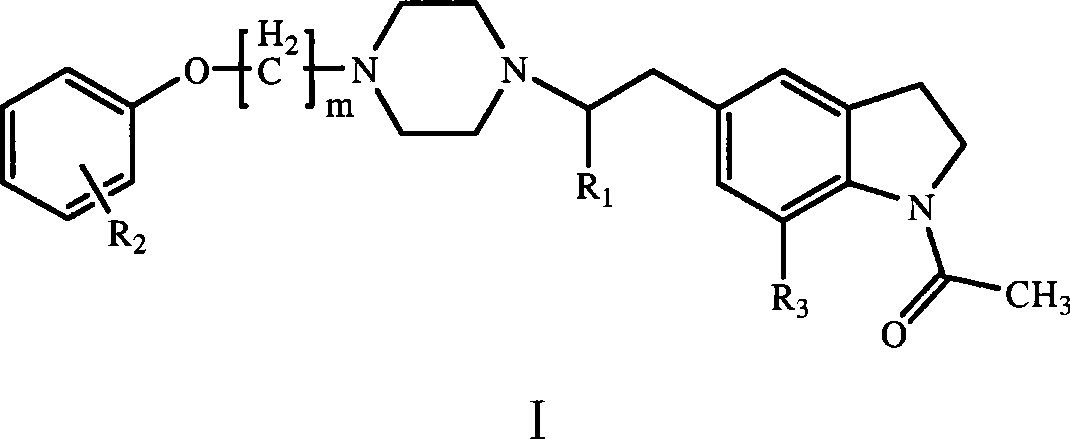
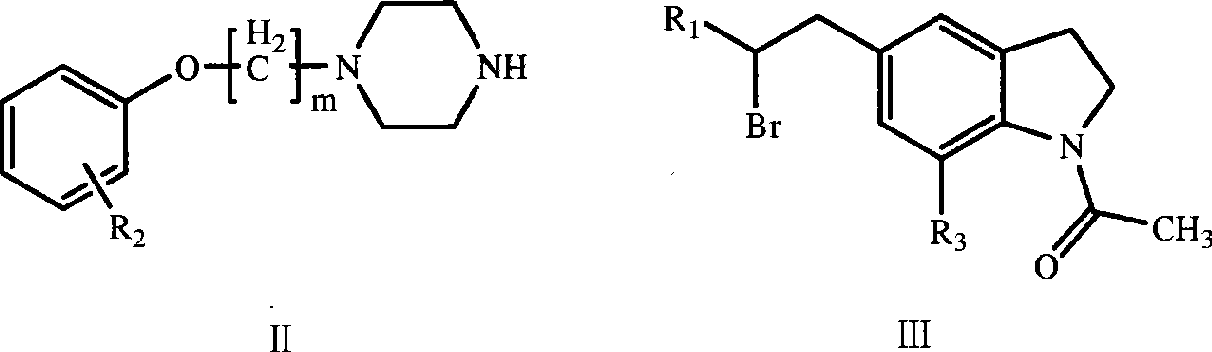
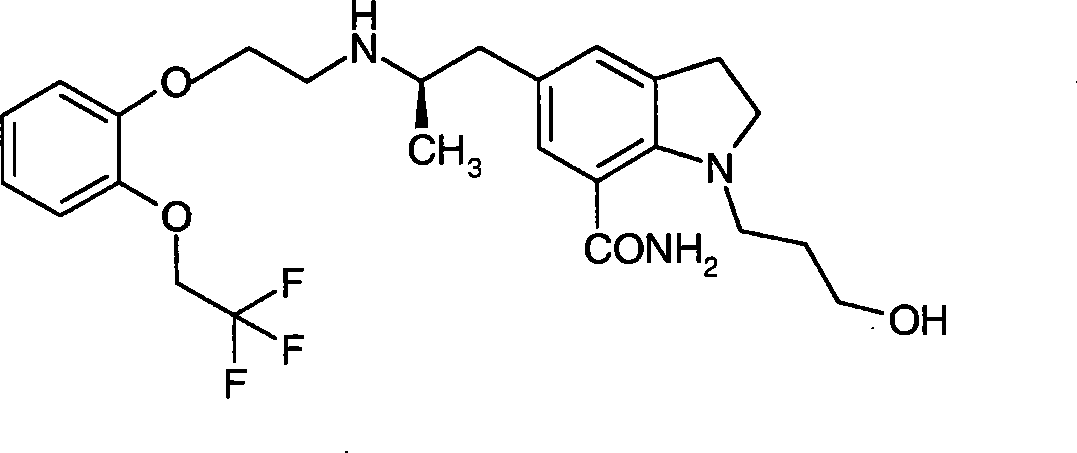
Indolines compounds, preparation method and pharmaceutical application thereof
A compound, indoline technology, applied in the field of indoline compounds, which can solve problems such as cardiovascular side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Example 1: 1-Acetyl-5-(2-bromopropionyl)indoline (VIa)
[0137] CH 2 Cl 2 90ml, anhydrous AlCl 3 100g (0.75mol), 37.5ml (0.36mol) of 2-bromopropionyl bromide were mixed and stirred, and 36.0g (0.22mol) of 1-acetylindoline (V) and CH 2 Cl 2 100ml of the prepared solution was added and reacted at room temperature for 6 hours. The reaction solution was poured into 1.0L ice water, with CH 2 Cl 2 250ml×3 extraction, combined with CH 2 Cl 2 layer, washed with water, anhydrous Na 2 SO 4 Dry, filter, and concentrate the filtrate under reduced pressure to obtain 54.0 g of light yellow solid, yield 82.6%, mp 138-141°C.
Embodiment 2
[0138] Example 2: 1-acetyl-5-(2-bromoacetyl)indoline (VIb)
[0139] V5.0g (0.03mol), 2-bromoacetyl bromide 10.0g (0.05mol), anhydrous AlCl 3 27.0g (0.20mol) and CH 2 Cl 2 40ml was prepared in a similar manner to VIa to obtain 8.0g of light yellow solid, yield 91.8%, mp 103-105.5°C.
Embodiment 3
[0140] Example 3: 1-acetyl-5-(2-bromopropyl)indoline (VIIa)
[0141] Add 50.0 g (0.17 mol) of VIa into 100 ml of TFA, stir at room temperature to dissolve, add 60 ml (0.38 mol) of triethylsilane dropwise, and react at room temperature for 12 hours. The reaction solution was distilled under reduced pressure, and the residual liquid was added to CH 2 Cl 2 150ml×3 extraction, combined organic layer, washed with water, anhydrous Na 2 SO 4 Dry, filter, and concentrate the filtrate under reduced pressure to obtain 45.5 g of off-white solid, yield 95.5%, mp 145-147°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com