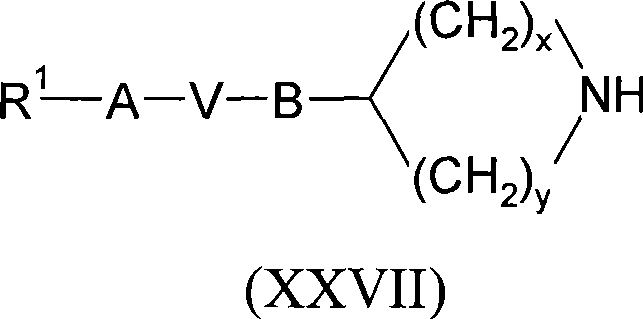Heterocyclic Gpcr agonists
A heterocyclic group, heteroaromatic ring technology, applied in the field of treatment of obesity and metabolic syndrome, GPR119 agonist, can solve problems such as insufficient treatment of dyslipidemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
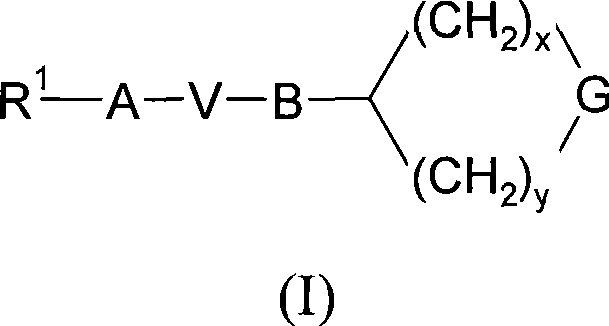
[0122] Further details on the preparation of compounds of formula (I) are found in the Examples.
[0123] Compounds of formula (I) can be prepared individually or as a compound library comprising at least 2, eg 5-1000 compounds of formula (I), more preferably 10-100 compounds of formula (I). Compound libraries can be prepared by combinatorial "split and mix" methods or multiple parallel syntheses using solution or solid phase chemistry using methods known to those skilled in the art.
[0124] In the synthesis of compounds of formula (I), labile functional groups such as hydroxyl, carboxyl and amino groups in intermediate compounds may be protected. Protecting groups may be removed at any step in the synthesis of compounds of formula (I), or present on the final compound of formula (I). An extensive discussion of the methods by which various labile functional groups can be protected and the methods for cleaving the resulting protected derivatives can be found, for example, in ...
Embodiment 1
[0221] Example 1: 4-{2-[3-(3-fluoro-4-methylthiophenyl)-[1,2,4]oxadiazol-5-yl]-1-methylethyl}piper tert-butyl pyridine-1-carboxylate
[0222]
[0223] A stirred solution of 3-fluoro-N-hydroxy-4-methylthiobenzamidine (200 mg, 1 mmol) in anhydrous THF (10 mL) was treated with sodium hydride (33.3 mg, 832 μmol). After 40 min, a solution of tert-butyl 4-(2-ethoxycarbonyl-1-methylethyl)piperidine-1-carboxylate (249 mg, 832 μmol) in anhydrous (3 mL) THF was added, and the mixture was heated under reflux for 18 h. The solvent was removed and the residue was purified by column chromatography (IH-EtOAc 4:1) to afford the title oxadiazole: δ H (CDCl 3 )0.99(d, 3H), 1.22-1.31(m, 2H), 1.39-1.45(m, 1H), 1.47(s, 9H), 1.68(br d, 2H), 2.08(m, 1H), 2.53( s, 3H), 2.66(m, 2H), 2.80(dd, 1H), 3.00(dd, 1H), 4.18(m, 2H), 7.31(t, 1H), 7.74(dd, 1H), 7.84(dd , 1H).
Embodiment 2
[0224] Example 2: 4-{2-[3-(3-fluoro-4-methylthiophenyl)-[1,2,4]oxadiazol-5-yl]propyl}piperidine-1-carboxy tert-butyl acid
[0225]
[0226] Using a method similar to that in Example 1, 3-fluoro-N-hydroxyl-4-methylthiobenzamidine was reacted with tert-butyl 4-(2-methoxycarbonylpropyl)piperidine-1-carboxylate , to obtain the title oxadiazole: RT=4.45min; m / z (ES + )=436.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com