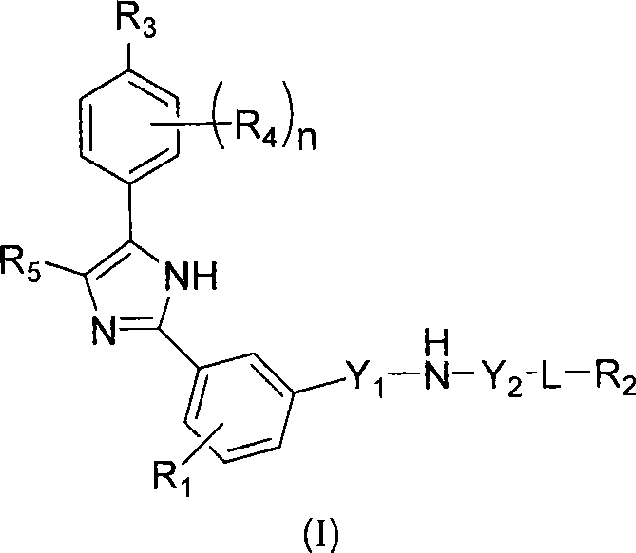
Compounds and compositions as HEDGEHOG signaling pathway modulators
A compound, selected technology, applied in the direction of drug combination, active ingredients of heterocyclic compounds, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
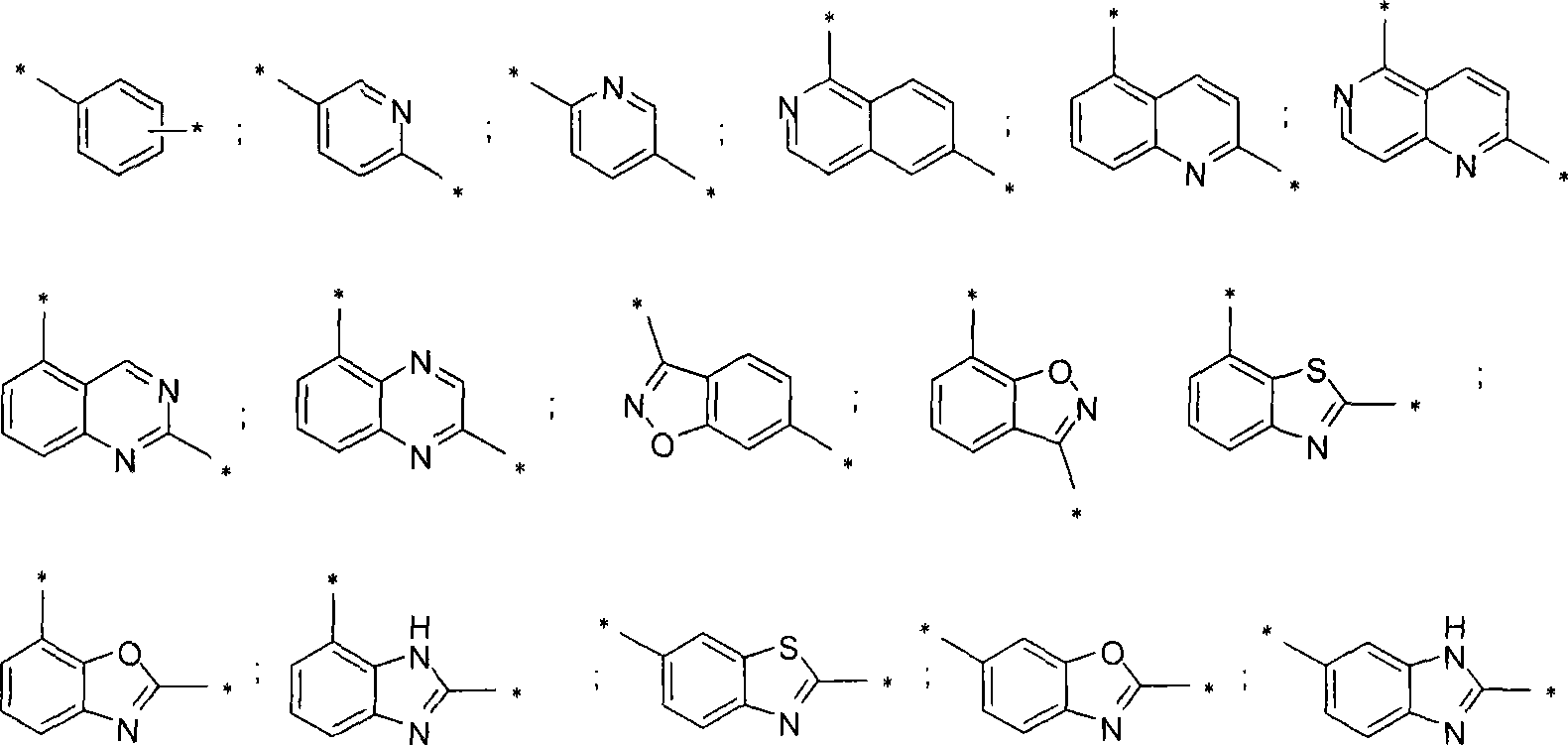
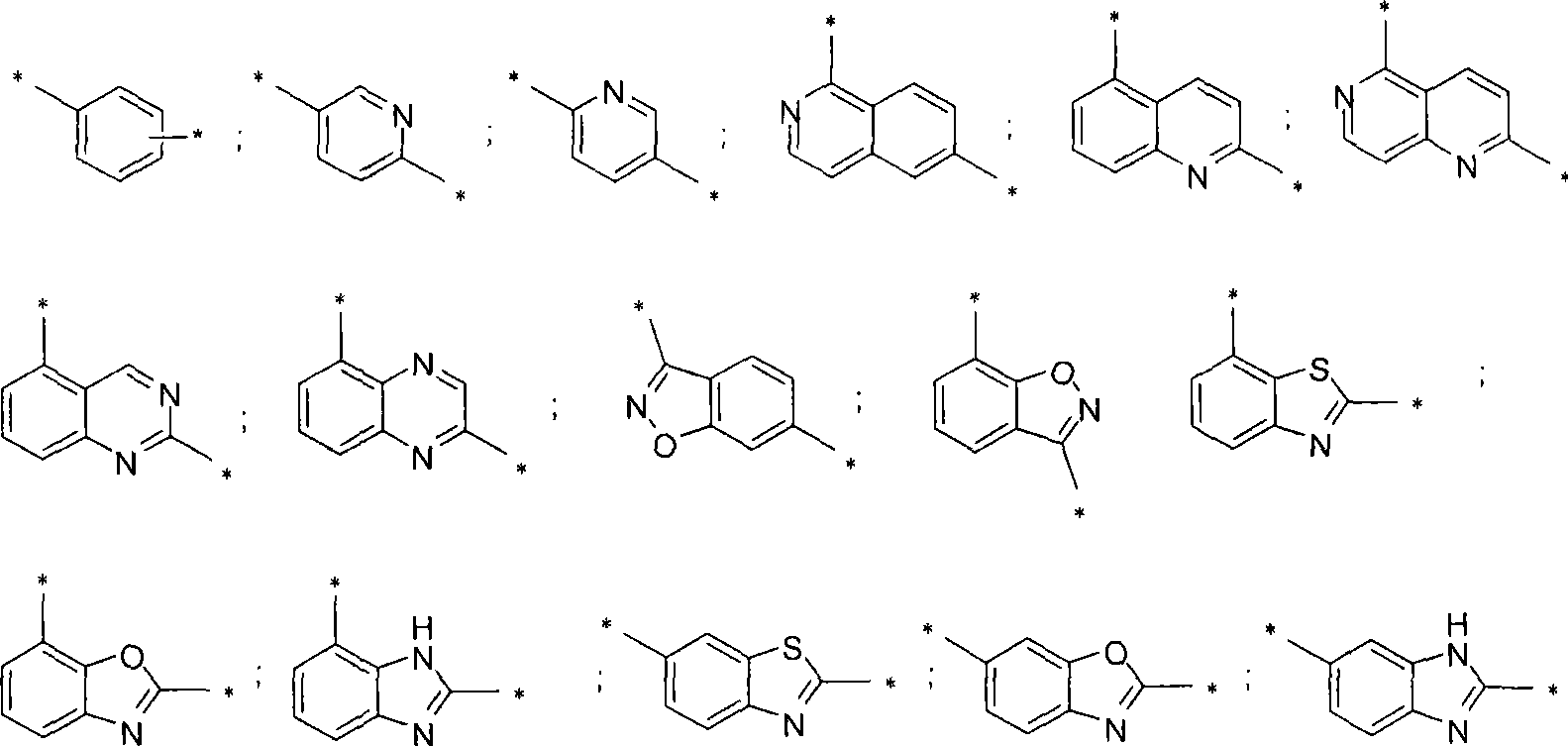
[0136] R-[4-Chloro-3-(5-phenyl-1H-imidazol-2-yl)-phenyl]-[6-(2-methyl-morpholin-4-yl)-isoquinoline-1 - Yl]-amine
[0137]
[0138] Step 1. R-2-Methylmorpholine hydrochloride:
[0139]
[0140] N-benzylethanolamine (9.06g, 60mmol) and (R)-(+)-propylene oxide (6.96g, 99%, 120mmol) were stirred overnight at 45°C in a sealed test tube. The excess propylene oxide was evaporated in vacuum to obtain a diol residue, which was used directly in the next step.
[0141] The above diol was dissolved in dioxane (60 mL, anhydrous). KOH (10.08g, 180mmol, powder) and tris(3,6-dioxaheptyl)amine (200mg, 0.62mmol) were added, the mixture was cooled to 0°C, and then tosyl chloride (12.58g, 66mmol, In 60mL anhydrous dioxane). The reaction mixture was stirred at 0°C for 45 minutes, then it was warmed to room temperature and stirred for another 4 hours. The reaction mixture was filtered (removal of insoluble materials, KCl, KOH), and the filtrate was evaporated in vacuo. HCl (2N, 200 mL) was adde...
Embodiment 2
[0153] R-[4-Chloro-3-(5-phenyl-1H-imidazol-2-yl)-phenyl]-[2-(2-methyl-morpholin-4-yl)-quinoline-5- base]- amine
[0154]
[0155] Step 1. Add 3-chloroperoxybenzoic acid (1.35g, up to 77%, ~1.5eq.) to a solution of 5-iodoquinoline (1.02g, 4.00mmol) in dichloromethane (20mL) at 0°C. ), the mixture was stirred until the reaction was complete (monitored by LC-MS). Then mix the mixture in DCM (100mL) and 10% Na 2 CO 3 Partition between solutions (2×50 mL). The organic phase was further washed with HCl (1N, 50 mL), a small amount of unoxidized quinoline derivative was extracted, and dried over sodium sulfate.
[0156] Step 2. Dissolve the obtained N-oxide in anhydrous DCM (15mL), and then add POCl 3 (920mg, 1.5eq.). The mixture was refluxed for 1 hour, cooled to room temperature, and then poured into Na at 0°C 2 CO 3 Solution (10% aqueous solution, 80 mL). After 30 minutes, it was diluted with DCM (50 mL), the organic phase was separated and dried over sodium sulfate. After evapor...
Embodiment 3
[0160] R-[4-Chloro-3-(5-phenyl-1H-imidazol-2-yl)-phenyl]-[2-(2-methyl-morpholin-4-yl)-[1,6] Naphthyridine-5- Yl]-amine
[0161]
[0162] Step 1. At 0°C, add 3-methyl-6H-[1,6]naphthyridin-5-one (prepared according to literature [3], 2.40g, 15.0mmol) in DCM (50mL) solution at 0°C. Chloroperoxybenzoic acid (5.50g, up to 77%, ~1.6eq.). After stirring for 1 hour at 0°C, the reaction mixture was evaporated, and the residue was directly dry-loaded on a short silica gel column and purified by chromatography (DCM: methanol (methonal) = 100: 6) to obtain 6- Methyl-1-oxy-6H-[1,6]naphthyridin-5-one.
[0163] Step 2. Combine the obtained N-oxide (2.20g, 12.5mmol) with POCl 3 (8.0 mL) was heated at 105-110°C until all the N-oxide solid was dissolved. Then it was cooled and left at 60°C over the weekend. After cooling it to room temperature, pour it into Na at 0℃ 2 CO 3 Solution (10% aqueous solution, 700 mL). After 30 minutes, it was extracted with DCM (250 mL×2), the organic phase was sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com