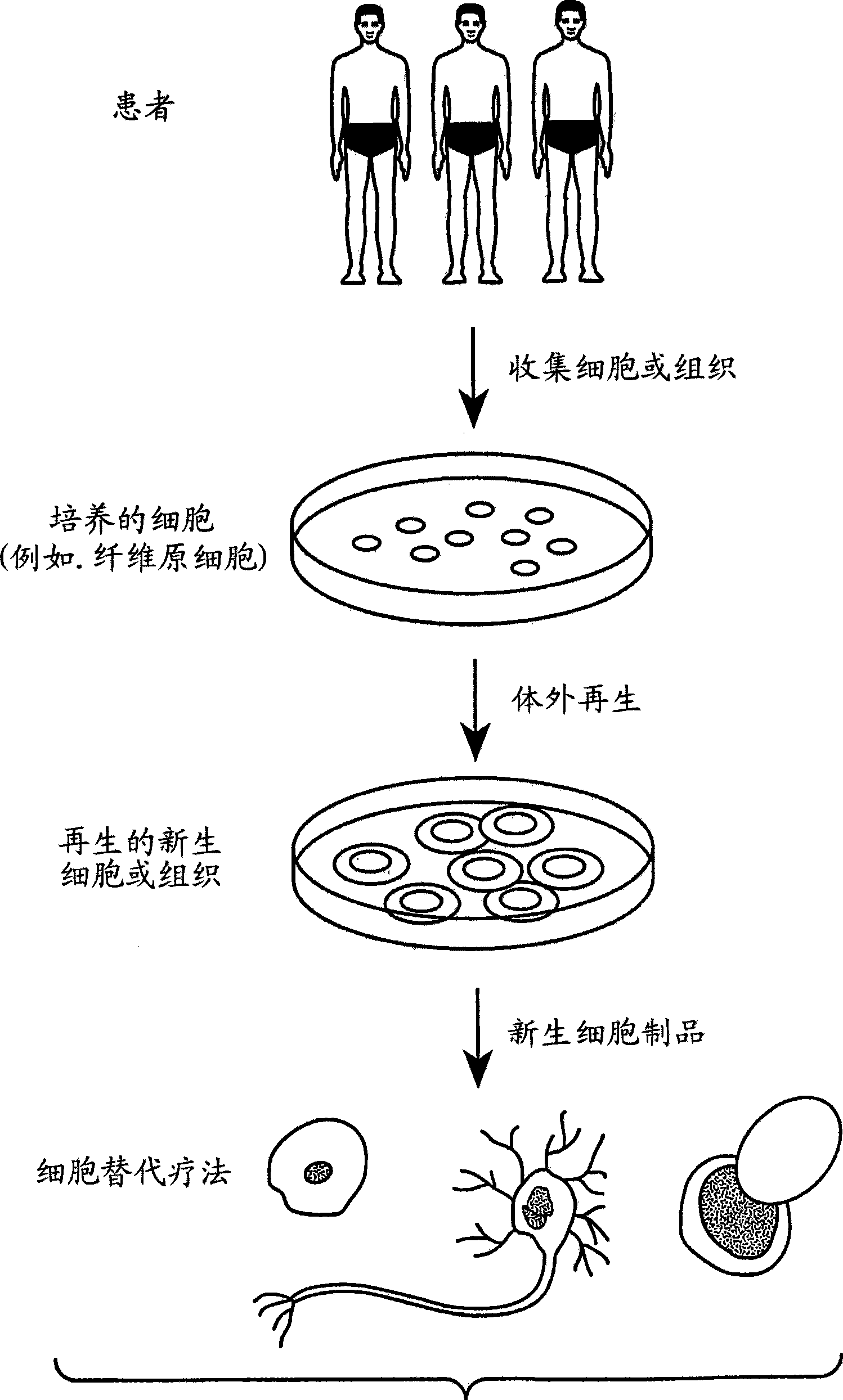
Methods for rejuvenating cells in vitro and in vivo
A technology of somatic cells and cells, applied in the field of embryonic stem cells or stem-like cells, can solve the problems of transdifferentiation of somatic cells and pluripotent cells that have not yet been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Embodiment 1-cultivate skin fibroblasts
[0082] After disinfection, a skin biopsy (2mm 2 ). Skin biopsies were cut into several small pieces with a sterile razor and placed directly into 6-well plates, in which a Thin-layer DMEM medium (Invitrogen, Carlsbad, California) covered it, and then supplemented 5% CO at 37°C 2 cultivated in indoor air. The medium was replaced daily with fresh DMEM.
[0083] After approximately 2 weeks in culture, fibroblasts had begun to grow around the edges of the skin. Fibroblasts were detached with 1X trypsin-EDTA (Invitrogen). The resulting trypsin / fibroblast solution was separated by centrifugation at 1200 rpm for 3 minutes. Resuspend the fibroblast pellet and count the cells. Depending on the count, cells were seeded in a new 6- or 24-well plate in DMEM medium. Fibroblasts were harvested and transferred to 75mm plates or flasks for further expansion. These aged fibroblasts grow more slowly and produce less collagen and elastin ...
Embodiment 2
[0084] Example 2 - Culturing Blood or Bone Marrow Cells
[0085] The success rate of bone marrow transplantation decreases with age, so one can reason that younger (neonatal) cells are preferred for hematopoietic reconstitution. Likewise, aging is also an important determinant of bone marrow stromal cell growth in cell culture. Stromal cells isolated from aged mice grew more slowly than those isolated from younger mice. Therefore, it is desirable to regenerate aged bone marrow cells in vitro before use in cell replacement therapy.
[0086] Leukocytes provide a rapid and routine source of terminally differentiated cells that can be used for in vitro regeneration. Blood samples of 10 mL were collected using sodium heparin as an anticoagulant and added to 15 mL tubes and diluted with four volumes of phosphate buffered saline (PBS) containing EDTA (3 mM). The diluted solution was loaded onto Ficoll-Hypaque medium (Sigma, St. Louis, MO) in 50 mL conical tubes and centrifuged wit...
Embodiment 3
[0087] Example 3 - Preparation of fetal extracts for use as regenerative factors
[0088] Tissues collected early in development (eg, fetuses and embryos) are excellent sources of regenerative factors for regenerating cells. The following example of mouse fetal liver illustrates this approach.
[0089] Fetuses were collected from pregnant mice, and fetal livers were dissected into petri dishes containing ice-cold PBS. Mince the liver tissue into small pieces with sterile scissors or a razor and transfer them to a glass homogenizer with PBS. Homogenize the liver tissue with gentle up and down movements of the pestle about 20 times. Cells were passed through a nylon layer to facilitate removal of fibrous connective tissue and centrifuged at 600 rpm for 10 minutes at 4°C. With ice-cold extraction buffer (50mM HEPES, pH7.4, 50mM KCl, 5mM MgCl 2 , 2mM β-mercaptoethanol, and 5mM EGTA) to wash the cells twice. Cells were washed with the same buffer additionally containing the fo...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap