Method and device for separation and depletion of certain proteins and particles using electrophoresis
A separation area, flow electrophoresis technology, applied in the direction of measuring devices, material analysis by electromagnetic means, analysis of materials, etc., can solve difficult electrophoretic separation/removal of analytes to maintain sufficient stability, no given, no instructions can be removed repeatedly High-abundance proteins and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
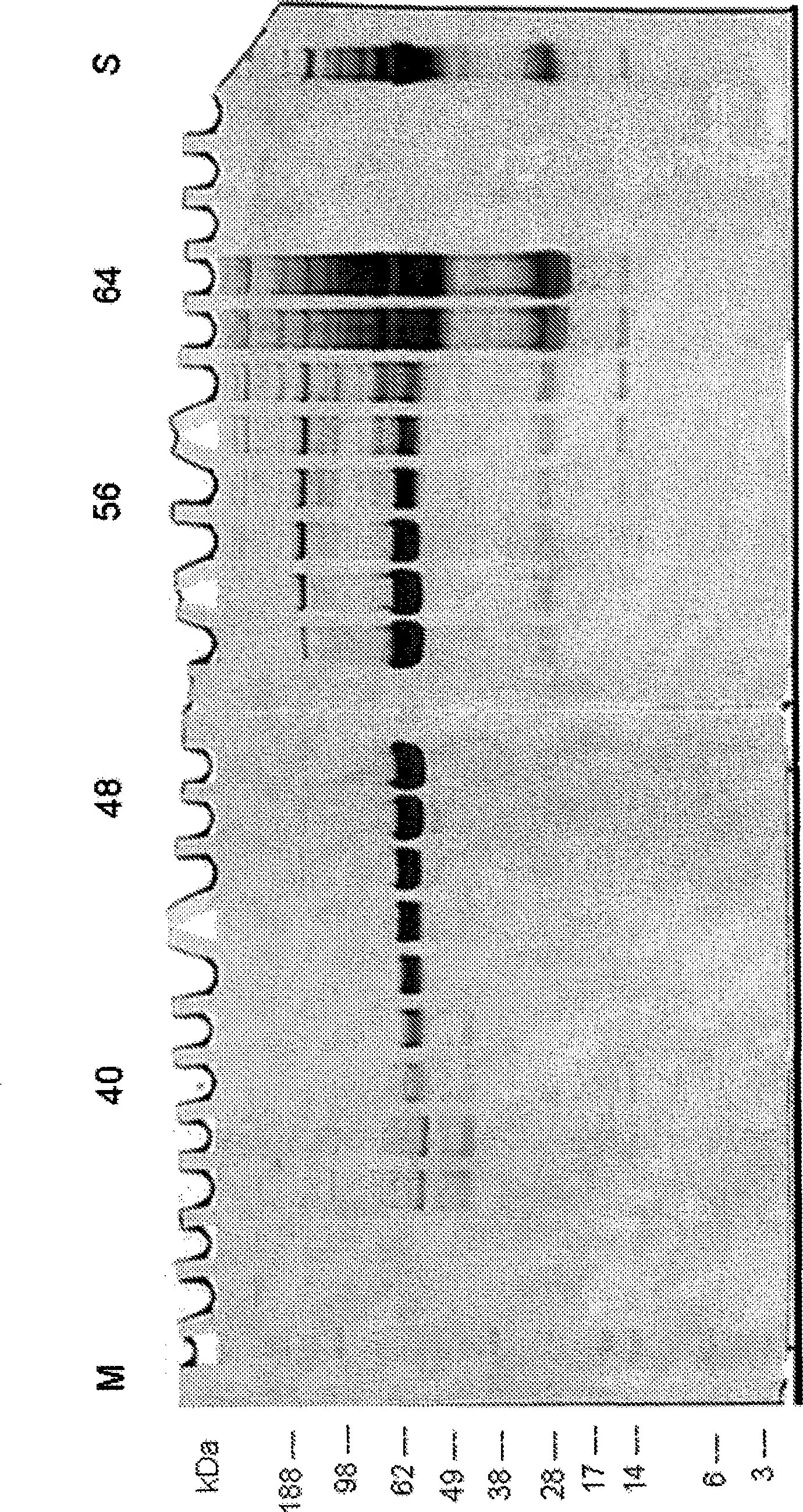
[0261] Example 1: Separation of human plasma according to the DFE protocol
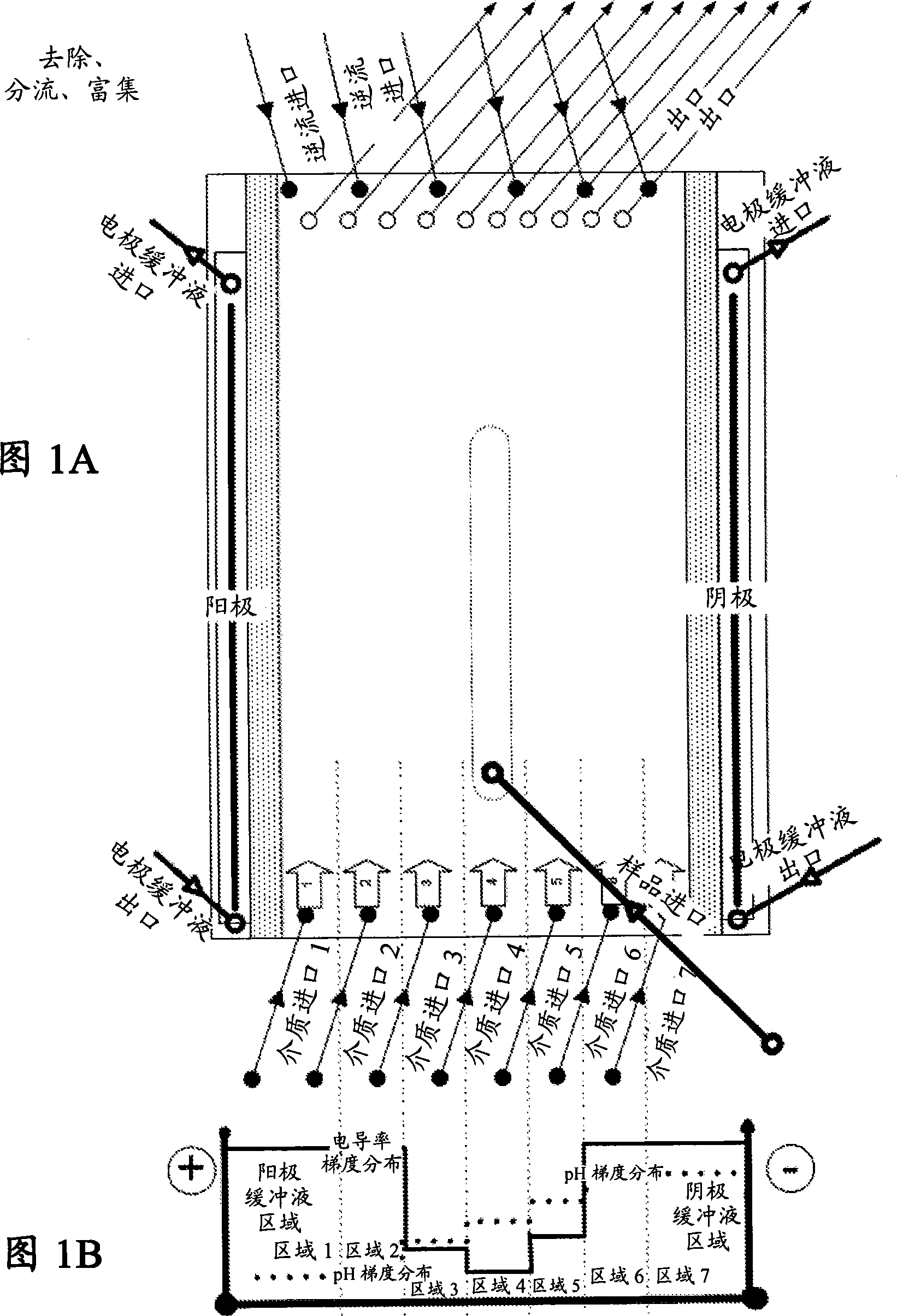
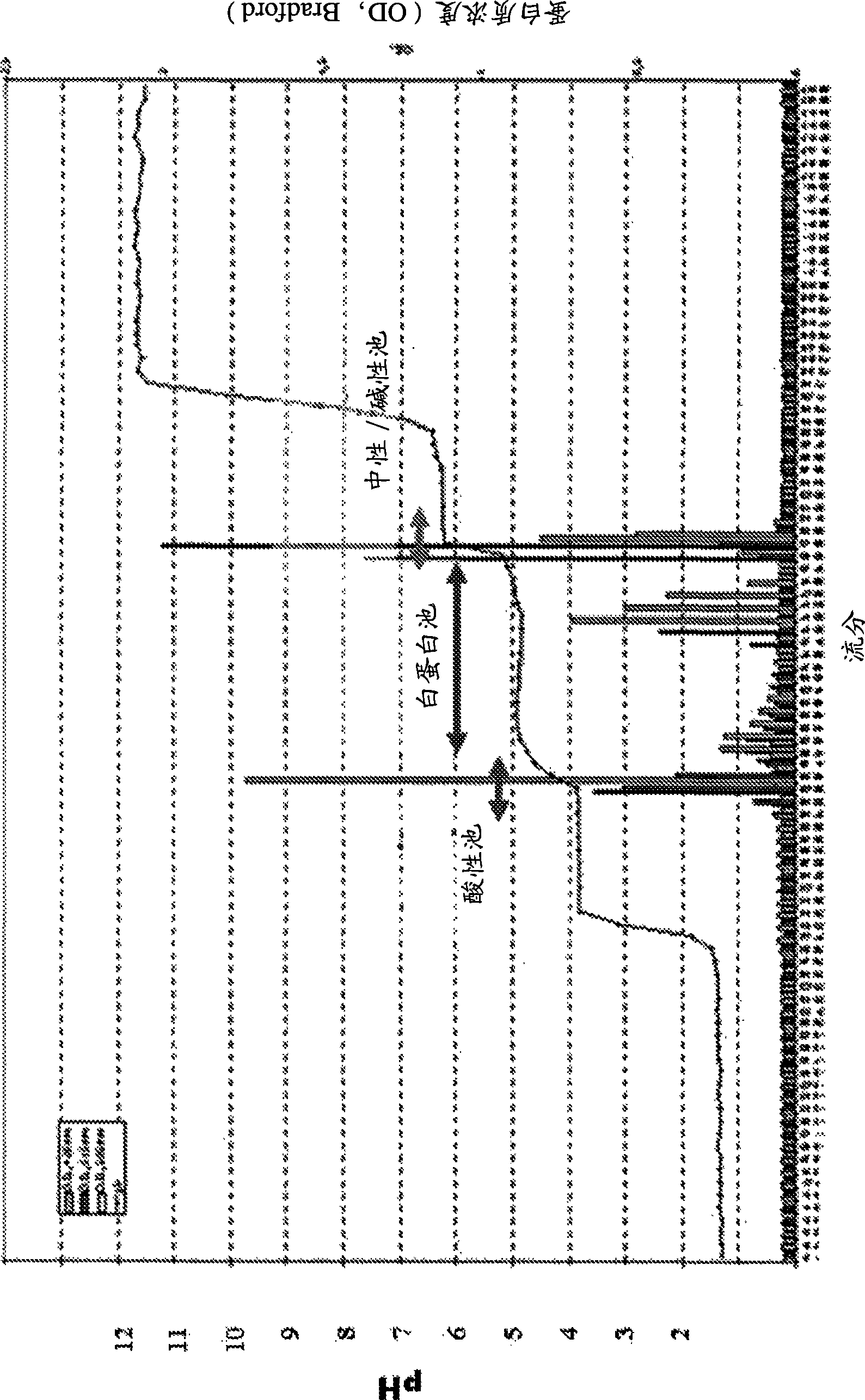
[0262] The examples illustrate the separation of high abundance proteins (human serum albumin, HSA, HSA) from human plasma samples using the DFE protocol using a gel-free, support-matrix-free or carrier-free FFE electrophoresis method and equipment suitable for carrying out the method. ). The non-denatured human plasma was diluted 1:10 with the medium of the medium inlet 4 of the device as shown in FIG. 1A and injected or introduced into the separation zone via the sample inlet 4 at a sample load rate of 5 ml / h.
[0263] Introduce the following media to the device:
[0264] Media inlet 1 and 2: 100mM sulfuric acid + 10% glycerol (pH1.30)
[0265] Media inlet 3: 200mM 2-amino-butyric acid, 100mM gluconic acid, 50mM pyridineethanesulfonic acid (PESS), 30mM glycylglycine, 10% glycerol (pH3.39)
[0266] Media inlet 4: 30mM MES, 100mM glycylglycine, and 10% glycerol (pH4.92)
[0267] Media inlet 5: 200...
Embodiment 2
[0276] Example 2: Separation of human plasma according to the DSE protocol
[0277] This example illustrates the separation of high-abundance proteins (human serum albumin, human serum albumin, HSA). The non-denatured human plasma was diluted 1:10 with the medium of the medium inlet 4 of the apparatus shown in Figure 4, and injected or introduced into the separation area through the sample inlet 4 at a sample loading rate of 5 ml / h.
[0278] Introduce the following media to the device:
[0279] Media inlet 1: 100 mM sulfuric acid; 10% glycerol
[0280] Media inlet 2: 100 mM sulfuric acid; 10% glycerol
[0281] Media inlet 3: 25% BD FFE Separation Buffer 1 + 10% Glycerol
[0282] Media inlet 4: 30 mM MES; 100 mM glycylglycine (glygly); 14% BDFFE separation buffer 2; 10% glycerol
[0283] Media inlet 5: 25% BD FFE Separation Buffer 2+10% Glycerol, (pH6.94)
[0284] Media inlet 6: 150mM NaOH+50mM ethanolamine, 10% glycerol
[0285] Medium inlet 7: 150mM NaOH+50mM ethanolam...
Embodiment 3
[0292] Example 3: Parallel DFE separation of analytes from two samples
[0293]This example illustrates the simultaneous separation of high-abundance proteins (human serum albumin, HSA, and ). The scheme adopted in this embodiment, starting from the anode to the cathode, employs the following media: anode stabilization media, a first separation zone containing a first pH function and a first pH separation plateau, an interelectrode stabilization media (which also acts as an adjacent separation zone 1 The focusing medium of the pH separation plateau of and 2), the second separation zone containing the pH separation plateau and the second pH function, and the cathode stabilization medium.
[0294] Dilute the first non-denatured human plasma sample 1:10 with the medium of medium inlet 2, and inject or introduce it into the separation area through the sample inlet located near the medium inlet 2 at a sample loading rate of 5 ml / h, while using the medium inlet 6 The medium dilute...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com