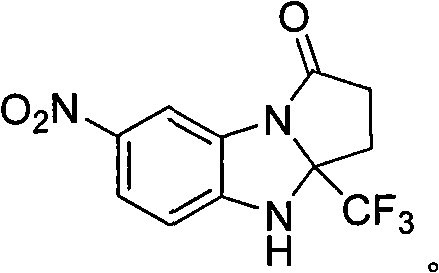
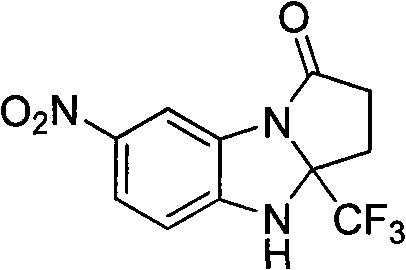
7-nitryl-3a-(trifluoromethyl)-2,3,3a,4-tetrahydrogen-1H-benzo(d) pyrrole (1,2-a) imidazole-1-ketone and synthesis method thereof
A technology of trifluoromethyl and p-toluenesulfonic acid, applied in the direction of organic chemistry, can solve the problems that have not been reported, and achieve high fat solubility and hydrophobicity, high drug efficacy, increased stability and physiological activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
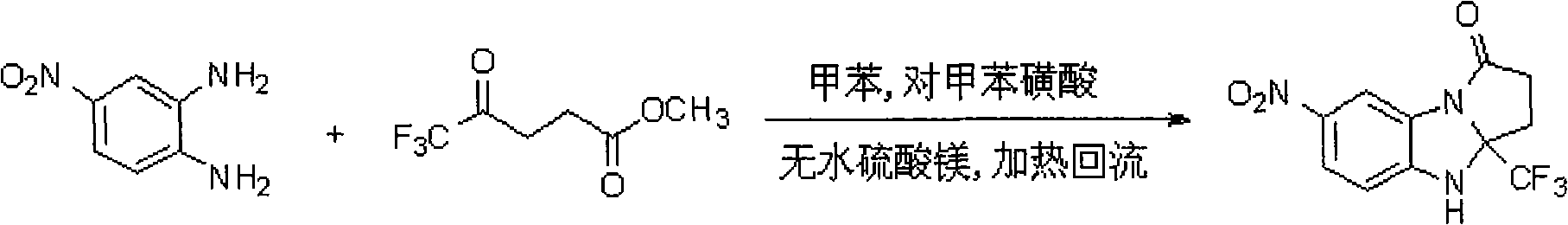
[0031] Embodiment 1: 1. add 0.44 grams of trifluoro γ-keto acid methyl esters, 0.019 grams of p-toluenesulfonic acid, 25 milliliters of toluene, 0.2 grams of anhydrous magnesium sulfate in 50 milliliters of round bottom flasks that reflux condenser tube is housed . The above mixture was stirred and refluxed in an oil bath for half an hour, and then 0.306 g of p-nitro-o-phenylenediamine was added; ② The reactant gradually turned dark red under reflux. After 12 hours of reaction, 0.019 g of p-toluenesulfonic acid was added. The reaction was continued for 24 hours and then stopped. After the reaction was completed, anhydrous magnesium sulfate was removed by suction filtration, and the filtrate was concentrated. ③ The obtained concentrate was separated by silica gel chromatography, and the developer was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 2:1 to obtain 0.25 g of a yellow solid with a yield of 43%.
Embodiment 2
[0032] Embodiment 2: 1. add 11 grams of methyl trifluoro γ-keto acids in a 250 milliliter round-bottomed flask equipped with a reflux condenser, 0.475 grams of p-toluenesulfonic acid, 150 milliliters of toluene, and 5 grams of anhydrous magnesium sulfate . The above mixture was stirred and refluxed in an oil bath for half an hour, and then 7.65 g of p-nitro-o-phenylenediamine was added; ② The reactant gradually turned dark red under reflux. After 16 hours of reaction, 0.475 g of p-toluenesulfonic acid was added. The reaction was continued for 32 hours and then stopped. After the reaction was completed, anhydrous magnesium sulfate was removed by suction filtration, and the filtrate was concentrated. ③ The obtained concentrate was separated by silica gel chromatography, and the developer was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 2:1 to obtain 5.87 g of a yellow solid with a yield of 41%.
Embodiment 3
[0033] Embodiment 3: 1. add 110 grams of trifluoro γ-keto acid methyl esters, 4.75 grams of p-toluenesulfonic acid, 1000 milliliters of toluene, 25 grams of anhydrous magnesium sulfate in 2 liters of round-bottomed flasks equipped with a reflux condenser . The above mixture was stirred and refluxed in an oil bath for half an hour, and then 76.5 g of p-nitro-o-phenylenediamine was added. ② The reactant gradually turned dark red under reflux. After 18 hours of reaction, 4.75 g of p-toluenesulfonic acid was added. The reaction was continued for 48 hours and then stopped. After the reaction was completed, anhydrous magnesium sulfate was removed by suction filtration, and the filtrate was concentrated. ③ The obtained concentrate was separated by silica gel chromatography, and the developer was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 2:1 to obtain 56.1 g of a yellow solid with a yield of 39%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap