Salts of 3-(4-amino-1-oxo-1,3-dihydro-iso-indol-2-yl)piperdine-2,6-dione and derivatives thereof, polymorph of the salts, preparation and application thereof
A technology of polymorphs and preparations, applied in 3-(4-amino-1-oxo-1,3-dihydroisoindol-2-yl)piperidine-2,6-dione and its Salts of derivatives or polymorphs of salts and their preparation and application fields can solve problems such as easy hydrolysis, compound instability, and no compound reports yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
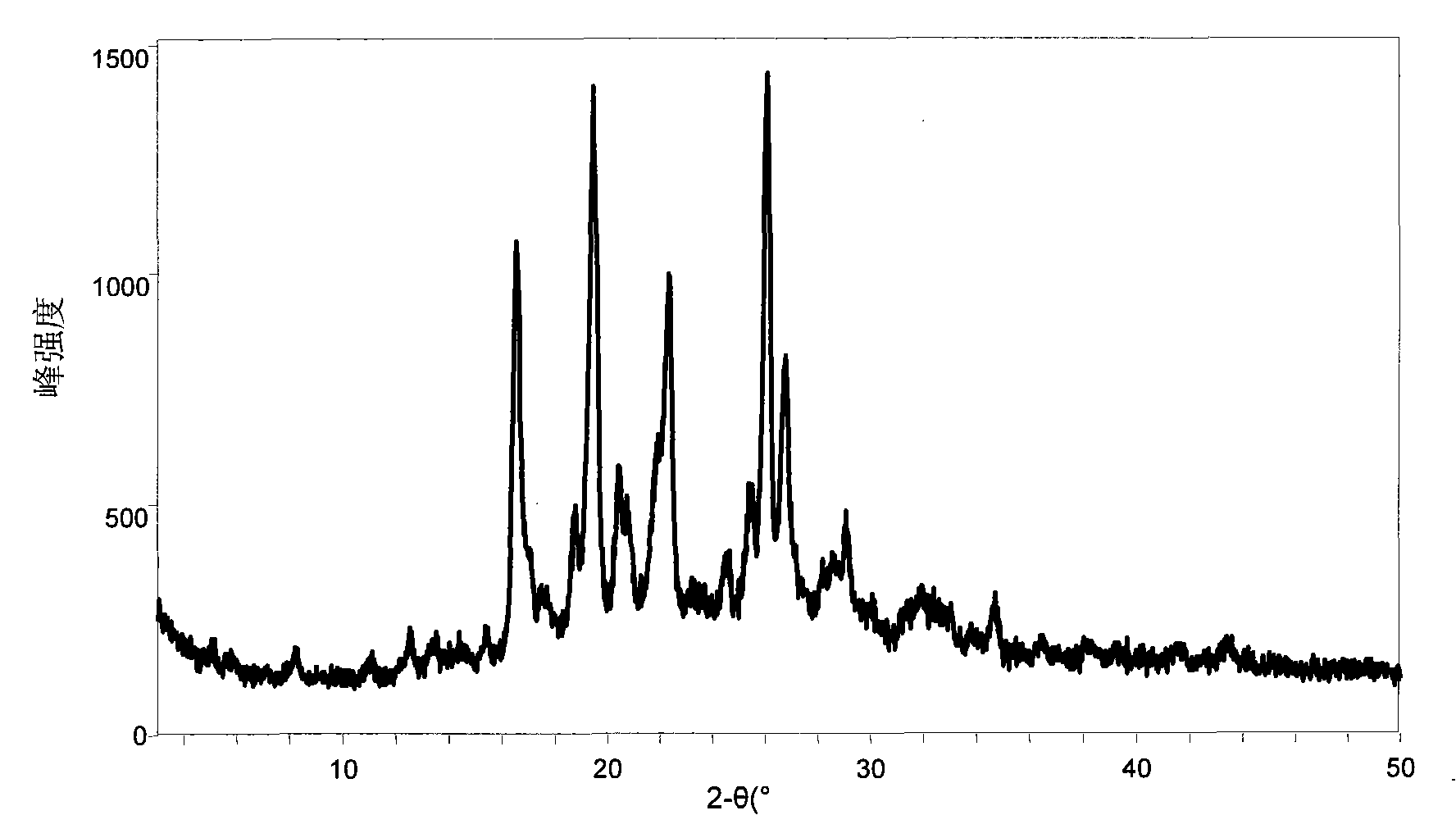
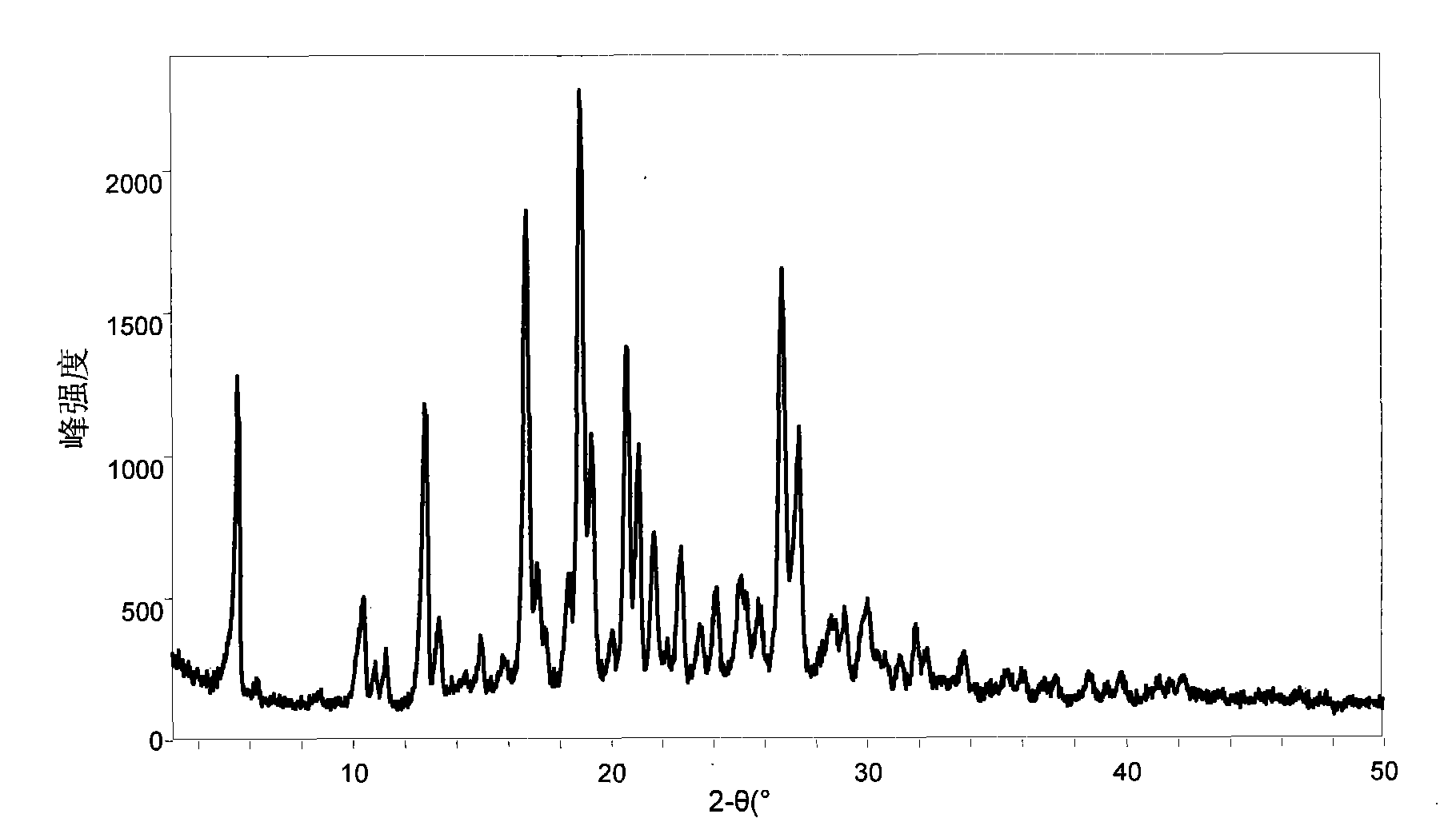
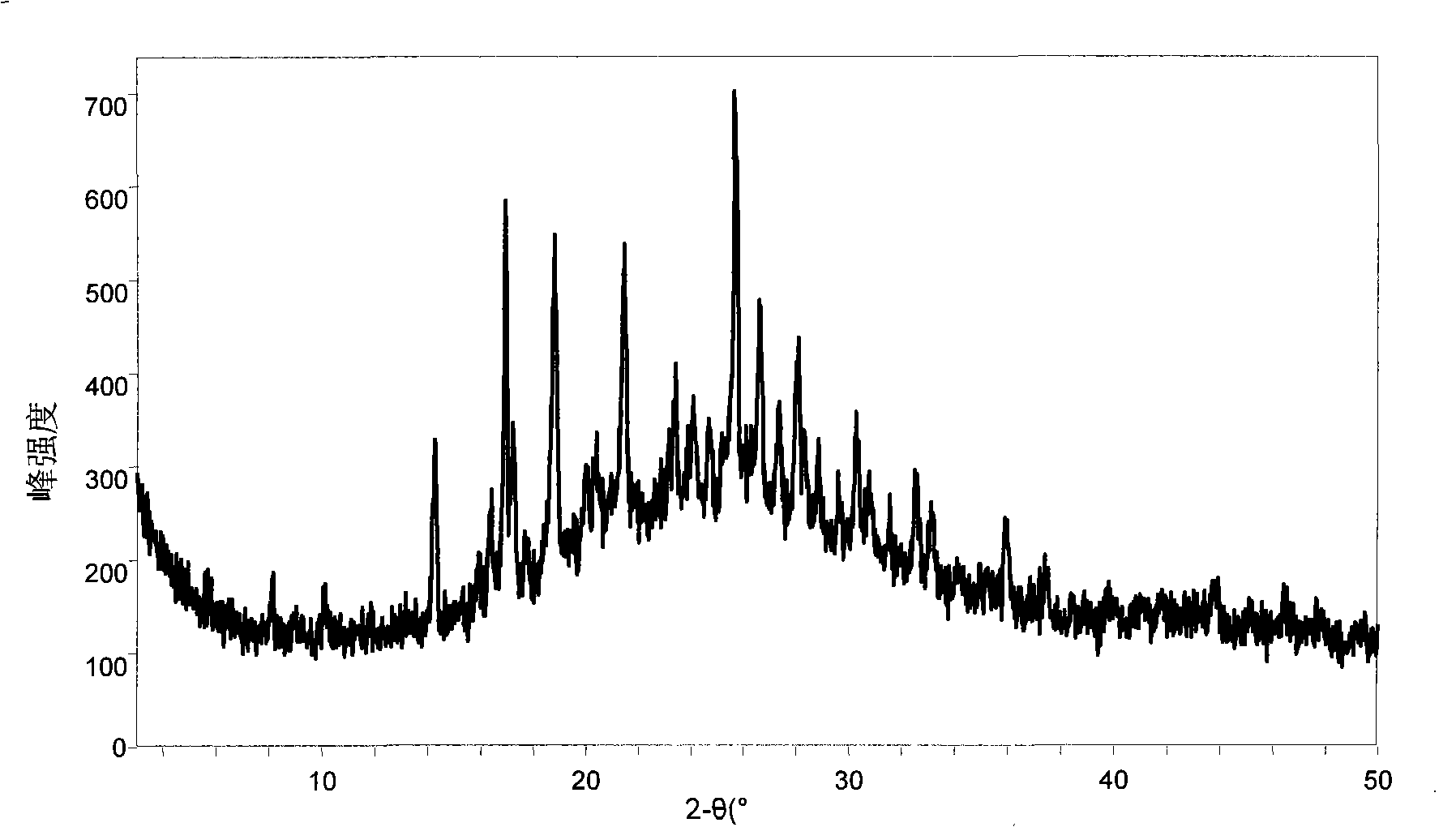
[0190] Take the compound (518 mg) represented by (II, where Y represents H) and an equimolar organic acid and add it to ethanol, heat and stir, add a small amount of water, continue stirring for 30 minutes, then stir at room temperature, and continue stirring for 4 hours after the solid is precipitated , suction filtration, and the filter cake was vacuum-dried for 10 hours. The water solubility of the obtained salt (1:1) is shown in Table 1.
[0191] Table one: (II, wherein Y represents the solubility of the organic acid salt of H) in water
[0192] Example Organic Acid Solubility (mg / mL, 15°C)
Embodiment 1
[0193] Embodiment 1 benzenesulfonic acid 3.76
Embodiment 2
[0194] Embodiment 2 methanesulfonic acid 1.85
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap