The use of granulin-epithelin precursor (GEP) antibodies for detection and suppression of hepatocellular carcinoma (HCC)
A hepatocellular carcinoma and antibody technology, applied in the direction of antibodies, immunoglobulins, animal/human proteins, etc., can solve the problems of limited targeted therapy, unsuitable treatment form, poor prognosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] patient sample
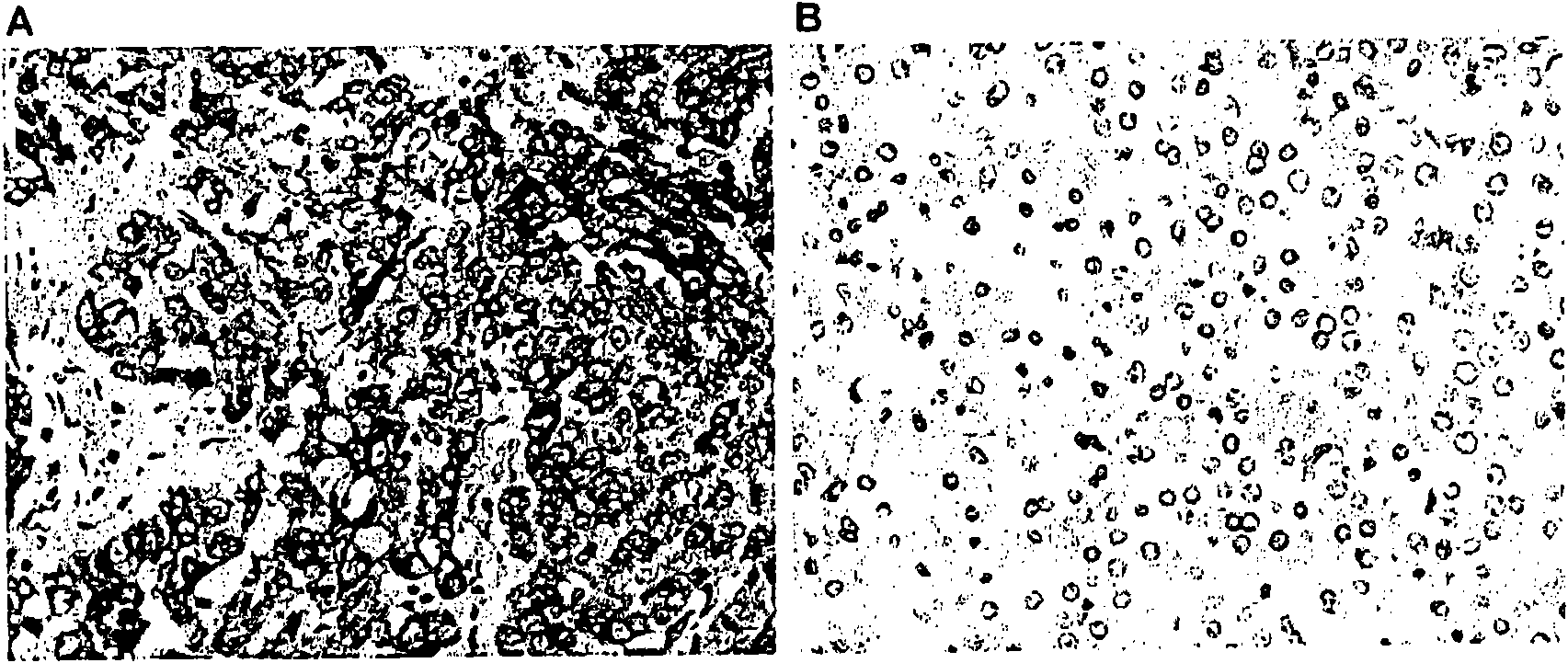
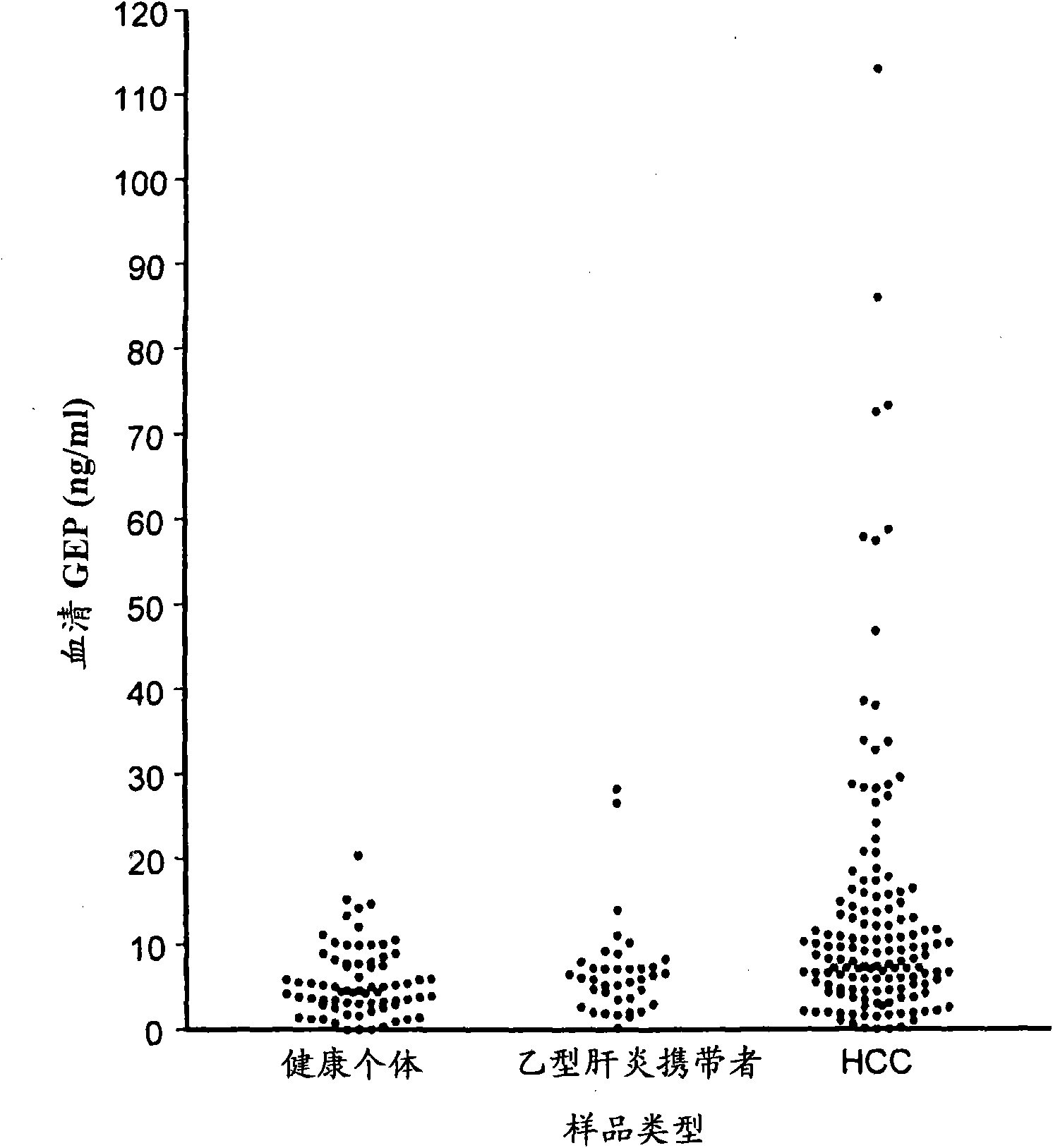
[0049] The study protocol was approved by the Institutional Review Board of The University of Hong Kong, and signed consent forms were obtained from patients and control subjects. Between March 1999 and October 2004, 107 patients diagnosed with primary HCC, 38 patients with chronic hepatitis B (only those with no signs of malignant disease during the follow-up period of more than 2 years) were included in this study) and 72 healthy donors negative for hepatitis B surface antigen (HBsAg) were obtained. Serum HBsAg was positive in 96 (89.7%) HCC patients, so the control group included chronic hepatitis B patients and healthy volunteers. Serum samples were frozen at -70°C until use. Tumor and adjacent non-tumor liver tissue were collected from HCC patients, snap frozen in liquid nitrogen, and stored at -70°C until use. Parallel sections were formalin-fixed and embedded in paraffin for histological examination and immunohistochemical studies. Clinical a...
Embodiment 2
[0051] cell line
[0052] Human HCC cell lines Hep3B, HepG2 and Huh7 (American Tissue Culture Collection (American Tissue Culture Collection), Manassas, VA) and Japan Health Science Research Resources Bank ((Japan Health Science Research Resources Bank), Osaka, Japan) ) were maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (Gibco BRL, Carlsbad, CA).
Embodiment 3
[0054] Build up antibodies
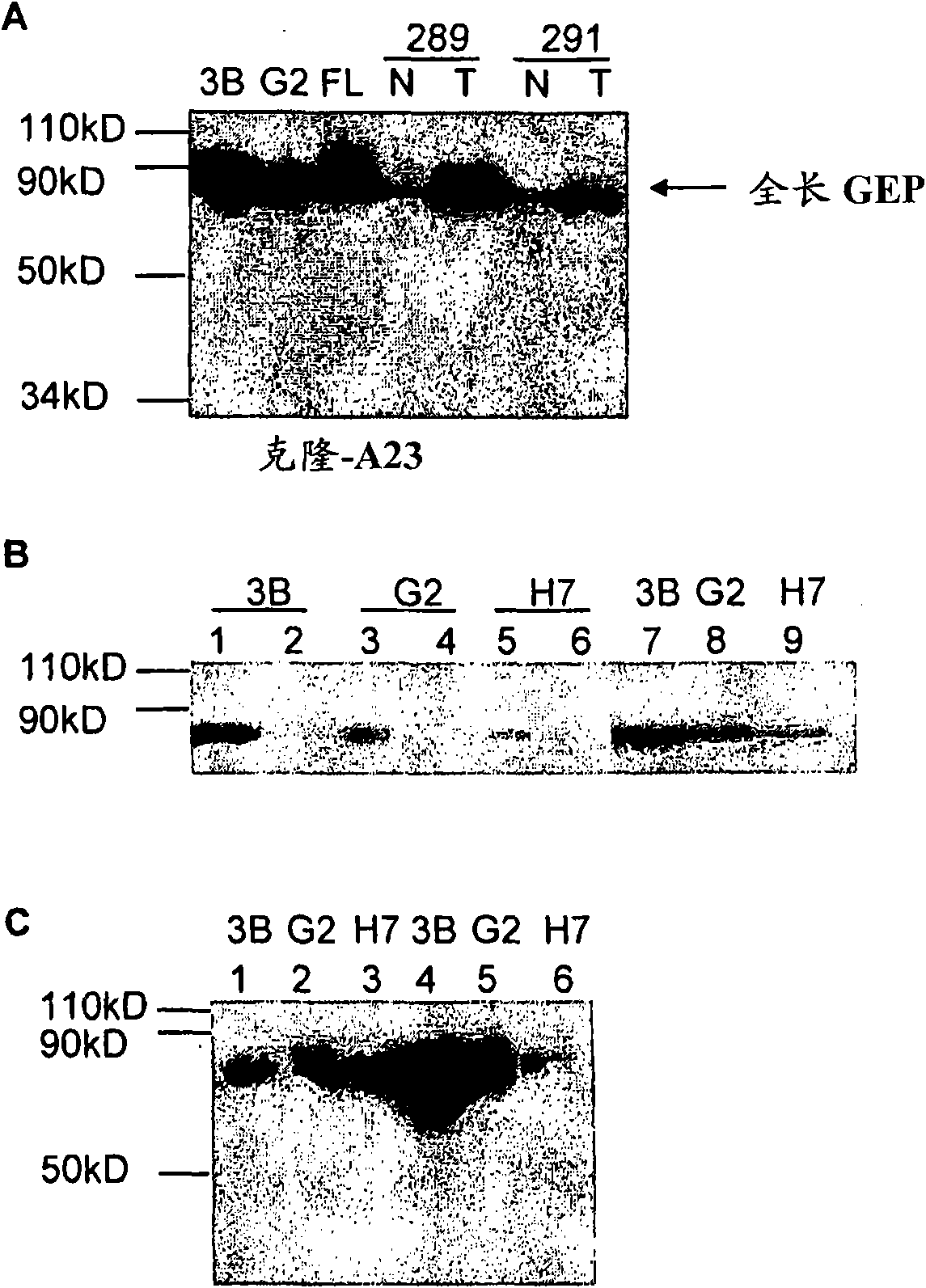
[0055] by conjugating the keyhole with 33 μg A custom GEP-specific peptide of hemocyanin (KLH), SEQ ID No: 3, was immunized subcutaneously in BALB / c mice with complete Freund's adjuvant (Sigma-Aldrich, Dorset, UK) to generate GEP-specific antibodies. For subsequent booster immunizations, the same amount of antigen in Freund's incomplete adjuvant was injected intraperitoneally twice a week. After each booster immunization, serum antibody activity against the immunizing antigen was monitored using an ELISA against the peptide antigen. Mice showing high serum antibody titers against the antigen were given a booster immunization with the last intravenous injection of the antigen, and spleens were harvested 3 days later.
[0056] Production of anti-GEP monoclonal antibody A23
[0057] Spleens were collected from mice showing high antibody titers in their sera. Fusion of splenocytes with the non-productive myeloma cell line NS0 was performed followi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com