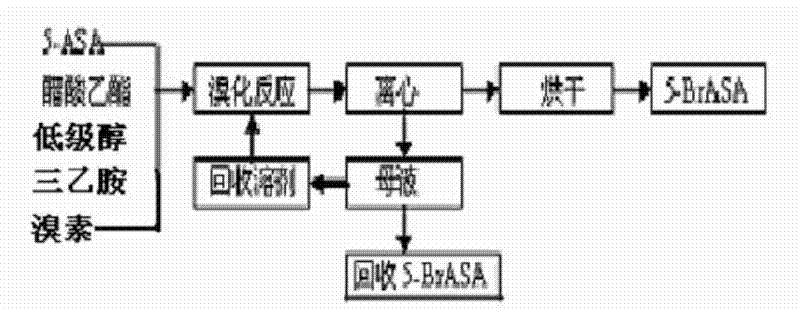
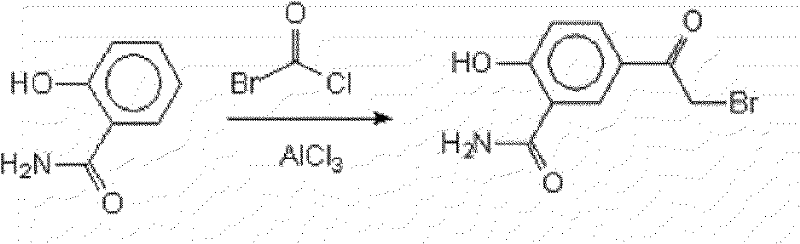
A kind of preparation method of 5-bromoacetyl salicylamide
A technology of bromoacetylsalicylamide and acetylsalicylamide, which is applied in the field of preparation of 5-bromoacetylsalicylamide, can solve problems such as being unable to meet the new requirements of 5-bromoacetylsalicylamide, achieves convenient and abundant raw material sources, The preparation method is simple and easy to implement, and the effect of low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
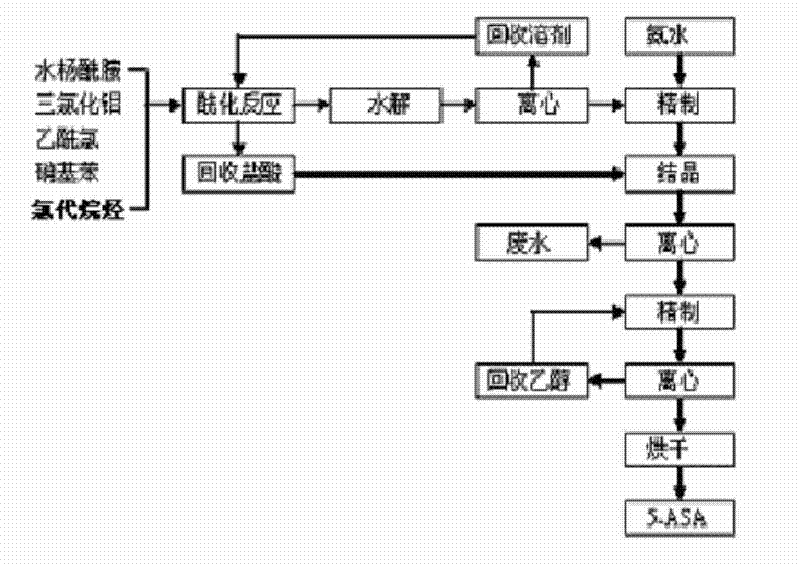
[0064] (1), 5-acetyl salicylamide (5-ASA) preparation
[0065] The acylation tank is vented and connected to the hydrochloric acid recovery unit. Under normal pressure, 1,000 kg of dichloroethane, 200 kg of nitrobenzene, and the freezing temperature is less than 10°C. Add 500 kg of aluminum trichloride, and then slowly add salicylamide 250 kg, slowly drop 145 kg of acetyl chloride (3 hours, temperature control is less than 10 ℃). It was then incubated at 20°C for 6 hours. Put 1200 kg of water and 100 kg of hydrochloric acid in the hydrolysis kettle, slowly put the material obtained from the above reaction into the hydrolysis kettle for hydrolysis, control the temperature to less than 60°C, then keep it at 60°C for 2 hours, and centrifuge to obtain the crude product 1. The mother liquor obtained by centrifugation is separated into layers, and the organic phase can be distilled to recover dichloroethane and nitrobenzene. Put crude product 1 into 1# refining kettle, put 1000 kg...
Embodiment 2
[0069] (1), 5-acetyl salicylamide (5-ASA) preparation
[0070] The acylation tank is vented and connected to the hydrochloric acid recovery device. Under normal pressure, 1200 kg of tetrachloroethane, 100 kg of nitrobenzene, and the freezing temperature is less than 20 °C. 250 kg, slowly drop 175 kg of acetyl chloride (5 hours, temperature control is less than 30 ℃). It was then incubated at 30°C for 4 hours. Put 1200 kg of water and 150 kg of hydrochloric acid into the hydrolysis kettle, slowly put the material obtained from the above reaction into the hydrolysis kettle for hydrolysis, control the temperature to less than 50°C, then keep the temperature at 50°C for 1 hour, and centrifuge to obtain the crude product 1. The mother liquor obtained by centrifugation is separated into layers, and the organic phase is distilled to recover tetrachloroethane and nitrobenzene. Put crude product 1 into 1# refining kettle, put 1000 kg of water, heat up to 50°C, slowly add 150 kg of am...
Embodiment 3
[0074] (1), 5-acetyl salicylamide (5-ASA) preparation
[0075] The acylation kettle is vented and connected to the hydrochloric acid recovery device. Under normal pressure, 1000 kg of dichloromethane, 600 kg of nitrobenzene, and the freezing temperature is less than 30°C. kg, slowly add 215 kg of acetyl chloride dropwise (2 hours, temperature control is less than 30°C). It was then incubated at 40°C for 3 hours. Put 1200 kg of water and 110 kg of hydrochloric acid into the hydrolysis kettle, slowly put the material obtained from the above reaction into the hydrolysis kettle for hydrolysis, control the temperature to less than 40°C, then keep the temperature at 40°C for 1 hour, and centrifuge to obtain the crude product 1. The mother liquor obtained by centrifugation is separated into layers, and the organic phase can be distilled to recover dichloromethane and nitrobenzene. Put crude product 1 into 1# refining kettle, put 1000 kg of water, heat up to 20°C, slowly add 100 kg ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com