Antibody compositions and methods for treatment of neoplastic disease
An antibody and monoclonal antibody technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, chemical instruments and methods, etc., can solve problems such as the weakening ability of tumor cells to transform
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0230] Screening of phage-displayed human Fabs against human IGF-I
[0231] Most, if not all, existing antibodies against IGF-II are not of human but usually of mouse origin. To develop human mAbs against IGF-II, we used a recently developed human mAb containing 10 10 A large library of native human Fabs from different phage-displayed Fabs. Recombinant human IGF-I was combined with dynal beads and used as target antigen for panning of antibody library. After three rounds of panning, 200 random individual phage clones were screened by phage ELISA using IGF-I as target. Of the clones that showed significant binding to IGF-I and were sequenced, 3 Fabs had unique sequence s; they were expressed in bacteria as soluble Fabs, purified, and tested for binding activity. Two Fabs designated m705 and m706 showed binding specificity for IGF-I only, whereas one Fab, m708, showed significant levels of binding to both IGF I and IGF II in ELISA and was identified by Selected for affinity ...
Embodiment 2
[0307] Inhibits phosphorylation of IGF-IR and insulin receptor
[0308] image 3 It was shown that IgG 708.2 inhibits the phosphorylation of IGF-IR in MCF-7 cells. MCF-7 cells were starved in serum-free medium for 6 hours, and then treated medium containing 1.5nM IGF-I or 10nM IGF-II and IgG708.2 at the indicated concentration was added. After 20 minutes the cells were cooled and lysed. IGF-IR was immunoprecipitated and phosphorylated receptors were detected using a phosphotyrosine-specific monoclonal antibody. The total amount of IGF-IR was detected by using the same polyclonal antibody as in immunoprecipitation.
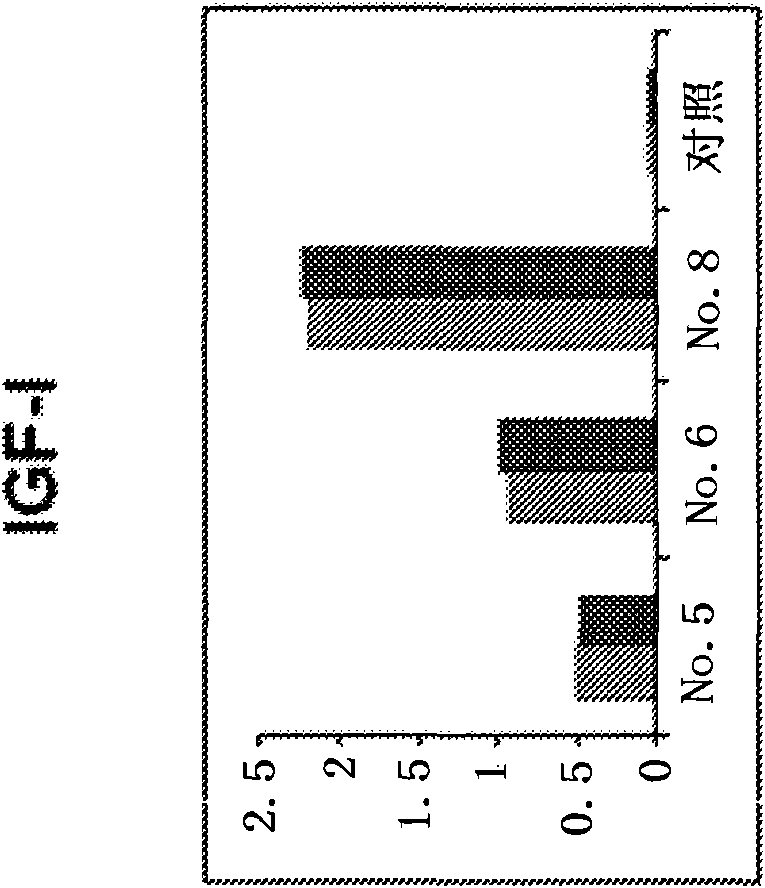
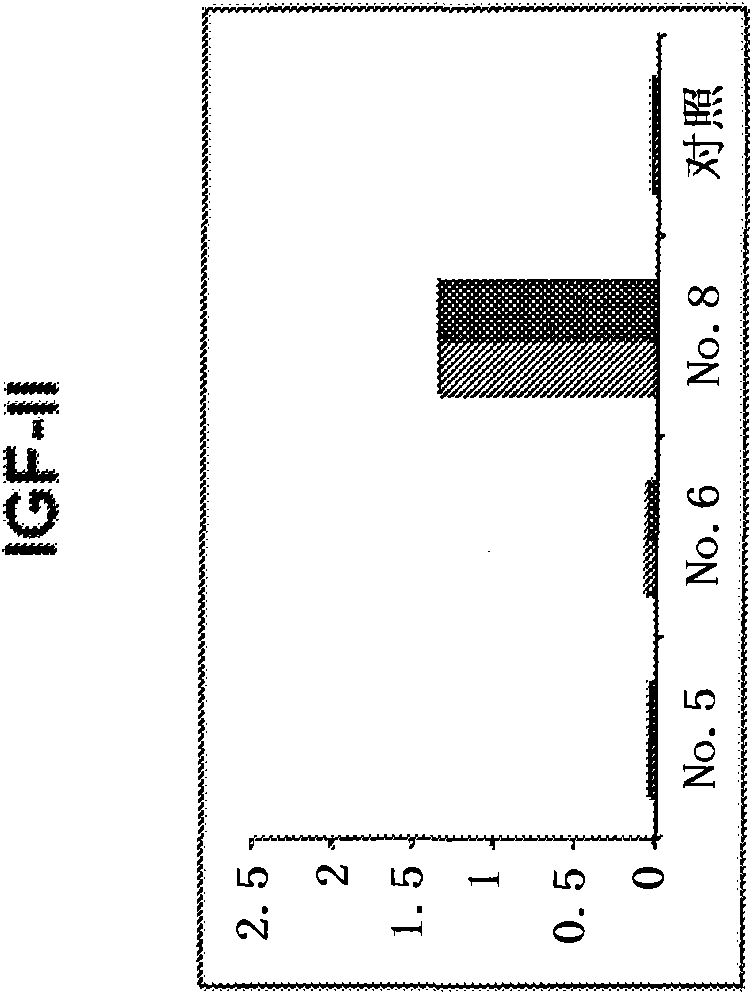
[0309] Figure 4 It is shown that human monoclonal antibodies screened against IGF-I inhibit the binding of IGF-I to soluble IGF-IR. The concentration of m705 and m708 was 40OnM. The concentration of m706 was 100 nM. The concentration of IGF-I was 50 nM and that of IGF-II was 500 nM.
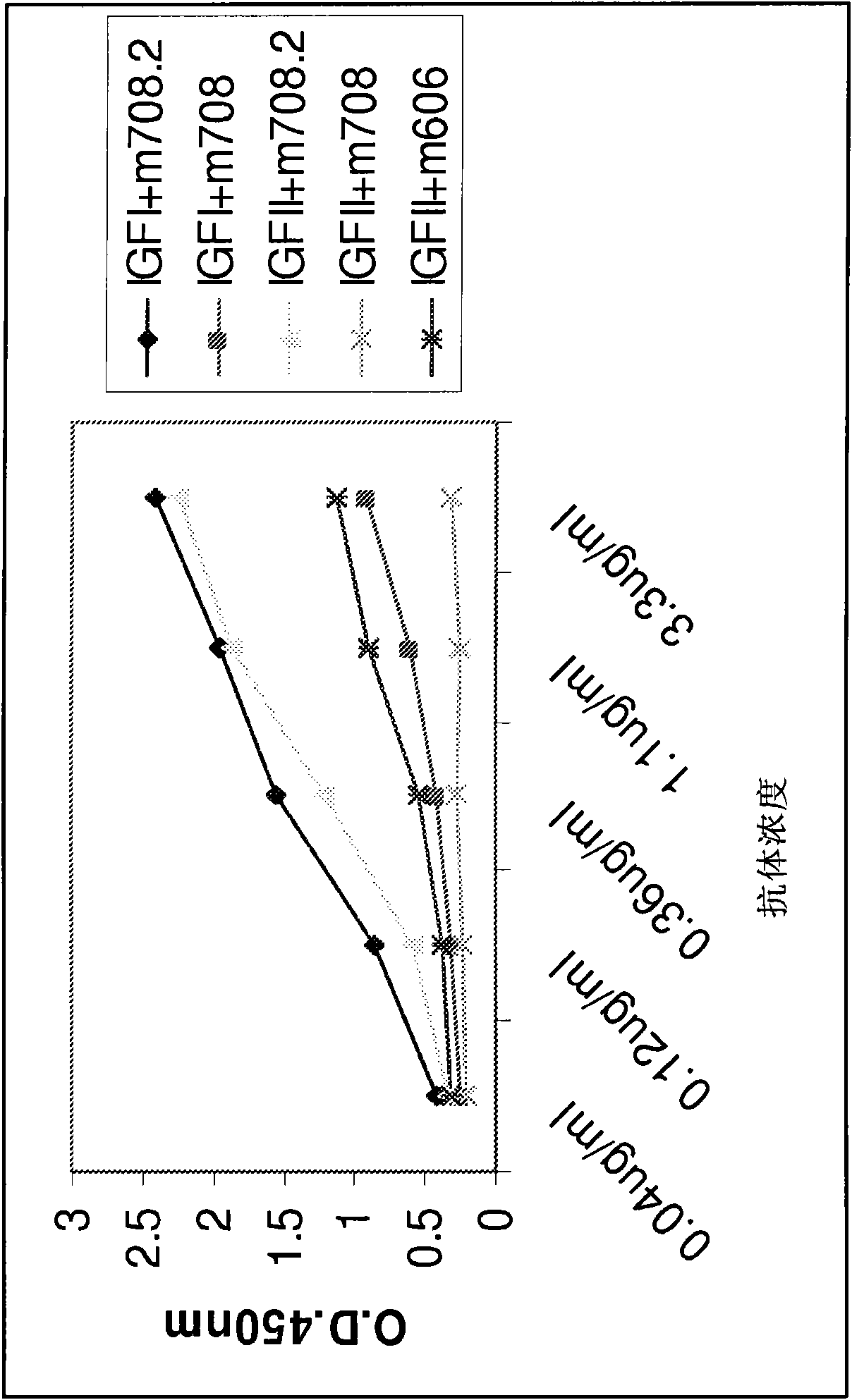
[0310] Figure 5 Dose-dependent inhibition of IGF-II and IGF-I-induced ...
Embodiment 3
[0317] Materials and Methods
[0318] Panning of phage display Fab libraries. Recombinant human IGF I was used to screen for cells containing 10 10 Human native Fab phage library of unique clones. Zhang et al., J. Virol. 78:9233-9242, 2004. Recombinant human IGF I was bound to magnetic beads and used as a target for library panning. 10 μg of antigen was used for the first round of panning. 10 12 The amplified phages were used for panning, and after washing, the bead-bound phages were used directly to infect exponentially growing TG1 cells and rescued with M13KO7 helper phage. Panning was repeated twice with 2 μg of antigen, and washed 10 times after each round. After the third round, 200 individual colonies were picked and inoculated into 2YT medium in 96-well plates for phage ELISA screening.
[0319] Generation and screening of light chain shuffled phage display libraries. The original human Fab phage display library was used as the source of the VL repertoire in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com