Bevantolol hydrochloride sustained release preparation
A technology of bevanolol hydrochloride and preparation, applied in the field of pharmaceutical preparations, can solve the problems of short plasma half-life and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
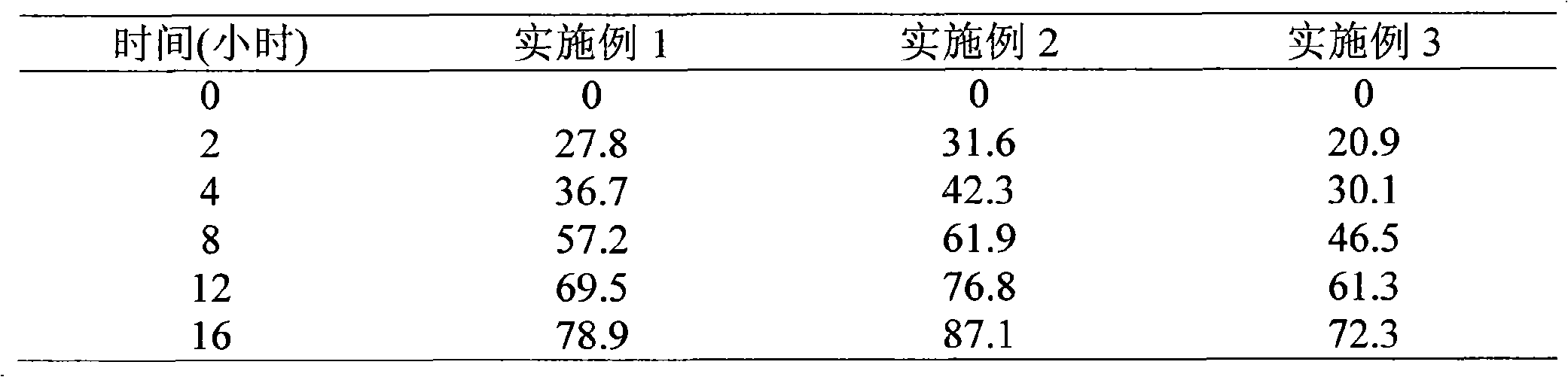
Embodiment 1 Embodiment 2 Embodiment 3
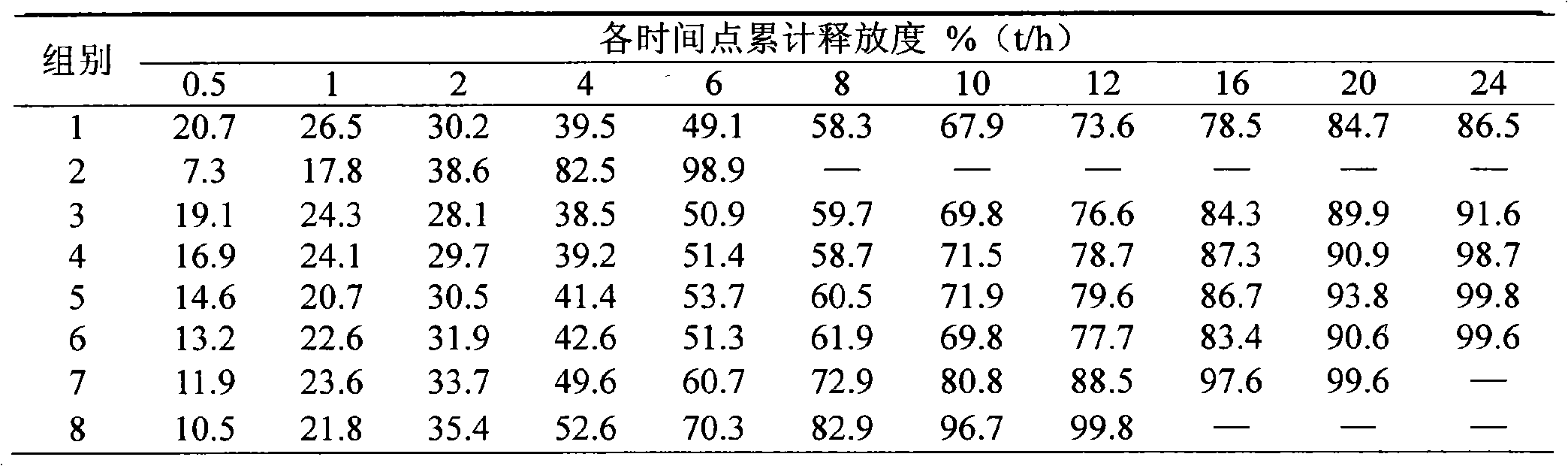
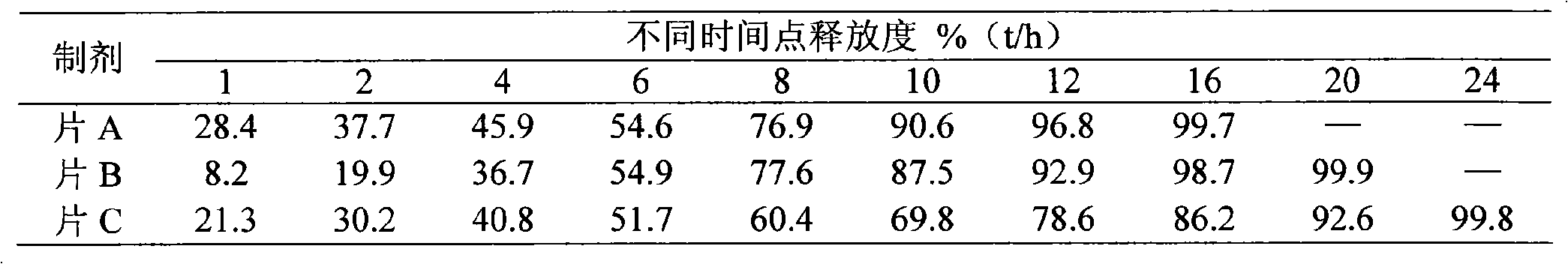
[0037]Bevanolol hydrochloride 100 50 100
[0038] Xanthan gum 102.9 60 133.3
[0039] HPMC K4M 17.1 15 16.7
[0040] Stearic acid 15 24 9
[0041] Microcrystalline cellulose 62 145 39
[0042] Magnesium stearate 3 6 2
[0043] Preparation process: the above-mentioned slow-release matrix xanthan gum, HPMC K 4M Disperse and dissolve with a sufficient amount of water respectively, add the prescribed amount of bevanolol hydrochloride and stearic acid into the aqueous solution of the sustained-release matrix, stir well, and form a uniform solution or dispersion, which is used as a spray-drying material liquid, and then sprayed Spray granulation is carried out in the dryer, the inlet temperature of the spray dryer is 125°C, the outlet temperature is 90°C, the feed rate is 8ml / min, the atomizer pressure is 125kpa, the particles are collected in the cyclone separator, and the average of the obtained particles is The particle size is 36.7 μm, for use;
[0044] The prepared bevano...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com