Contrast medium of dual-sector targeted magnetic resonance imaging contrast and preparation thereof
An MRI and contrast agent technology, applied in the direction of MRI/MRI contrast agent, etc., can solve the problems of high injection dose and injection frequency, short stay time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
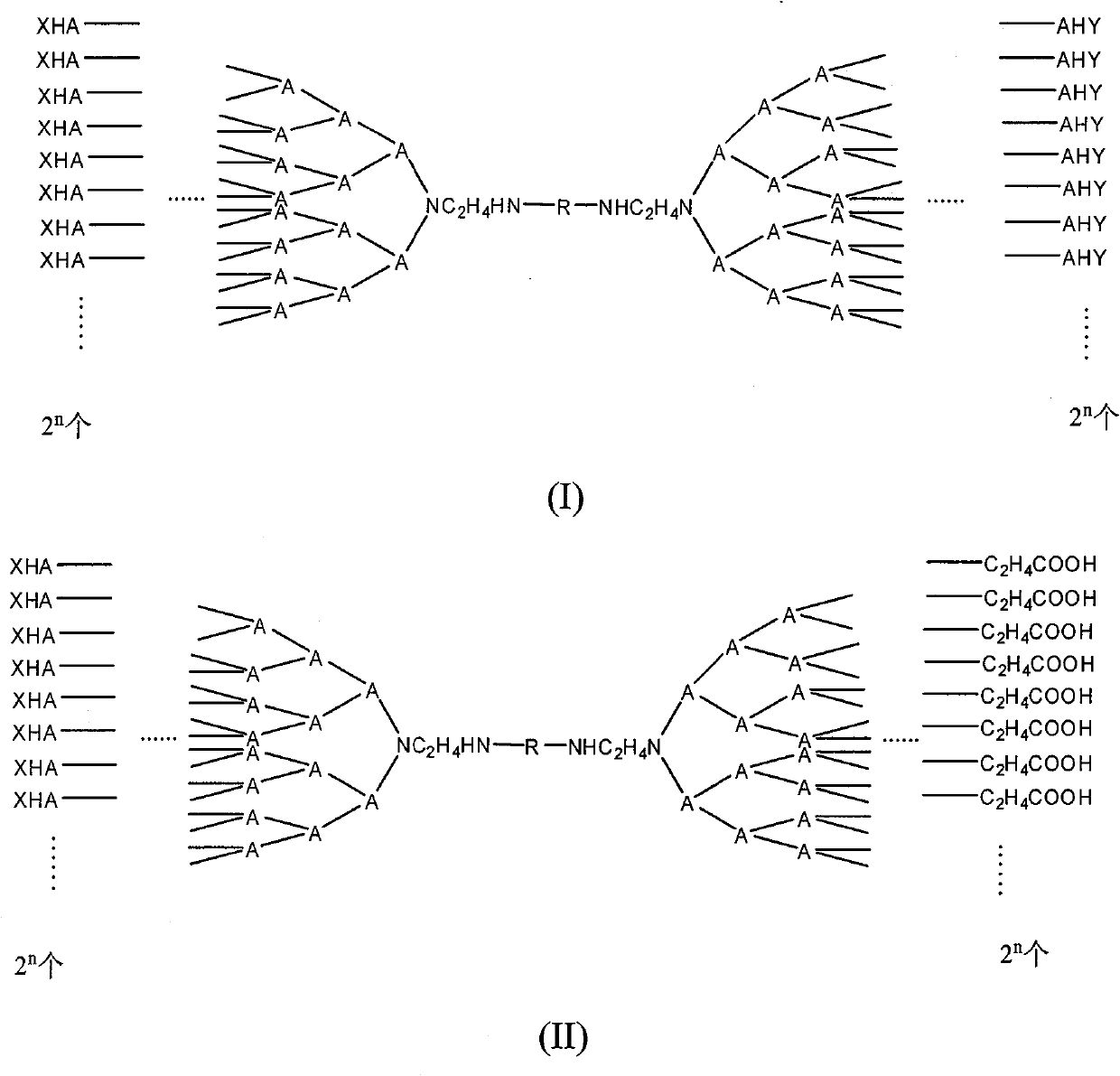
[0051] 1) Synthesis of sector-shaped parts containing small molecule ligands:
[0052] a: Weigh 0.1mol of ethylenediamine and dissolve it in 50ml of dichloromethane, add it to a 250ml there-necked flask, and slowly add dropwise 50ml of the dichloromethane solution that dissolves 0.1mol of di-tert-butyl dicarbonate (BOC anhydride) under stirring, The reaction was carried out at room temperature for 12 hours. Using dichloromethane:methanol=3:7 (V / V) as the eluent, the ethylenediamine protected at both ends was separated and removed by silica gel column chromatography, and the ethylenediamine product protected at one end was retained.
[0053] b: the above-mentioned product of 0.05mol was dissolved in 15ml of methanol, added to a 100ml there-necked flask, slowly dripped with 25ml of methanol solution dissolved with 0.4mol of methyl acrylate, stirred at room temperature for 12 hours, and the solvent methanol and excess were removed by rotary evaporation of methyl acrylate to give...
Embodiment 2
[0067] 1) Synthesis of sector-shaped moieties containing small molecule ligands
[0068] a: Synthesis of compounds containing 8 terminal ester groups. Same as 1) a, b, and c in Example 1, and repeat steps b and c until a compound containing 8 terminal ester groups is synthesized.
[0069] b: Take 10 mmol of the compound containing 8 terminal ester groups in 10 ml of water, add 0.1 mmol NaOH, react at 50 °C for 12 hours, dialyze with a dialysis membrane with a molecular weight of 500 for 24 hours, and freeze-dry to obtain a protective group containing 8 terminal carboxyl groups. Dendrimers.
[0070] c: Dissolve 1.0 mmol of the product obtained in step b in 10 ml of dichloromethane solvent, add 1.0 ml of 1M hydrochloric acid to the solution, and react at 35° C. for 12 hours (TLC tracks the reaction progress) to remove the BOC protecting group. After the reaction was completed, the reaction mixture was purified by column chromatography.
[0071] 2) Synthesis of sector part conta...
Embodiment 3
[0083] 1) Synthesis of sector-shaped parts containing small molecule ligands:
[0084] Same as Example 1.
[0085] 2) Synthesis of sector part containing targeting group:
[0086] Same as Example 1.
[0087] 3) Preparation of double sector compounds
[0088] Dissolve 0.8 mmol of the product obtained in 1) in 5 ml of DMSO, slowly add 0.8 mmol of adipoyl chloride dropwise with stirring, react at room temperature for 2 hours, and use a dialysis bag with a molecular weight cut-off of 500 to dialyze against deionized water for 12 hours. The dialyzed product was purified by column chromatography, and the adipoyl chloride product with only one end reacted was retained.
[0089] 0.6 mmol of the product was dissolved in 5 ml of DMSO, 2) 0.5 mmol of the product was slowly added dropwise with stirring, and the reaction was carried out at room temperature for 2 hours to obtain a double fan-shaped dendrimer. The reaction mixture was dialyzed against deionized water for 24 hours and lyo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com