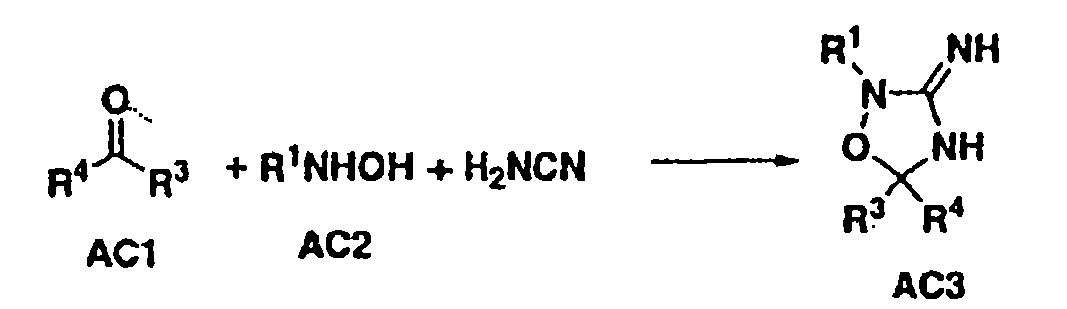Heterocyclic aspartyl protease inhibitors
A heteroaryl and solvate technology, applied in the field of heterocyclic aspartyl protease inhibitors, can solve problems such as not meeting medical requirements and not being able to prevent disease progression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
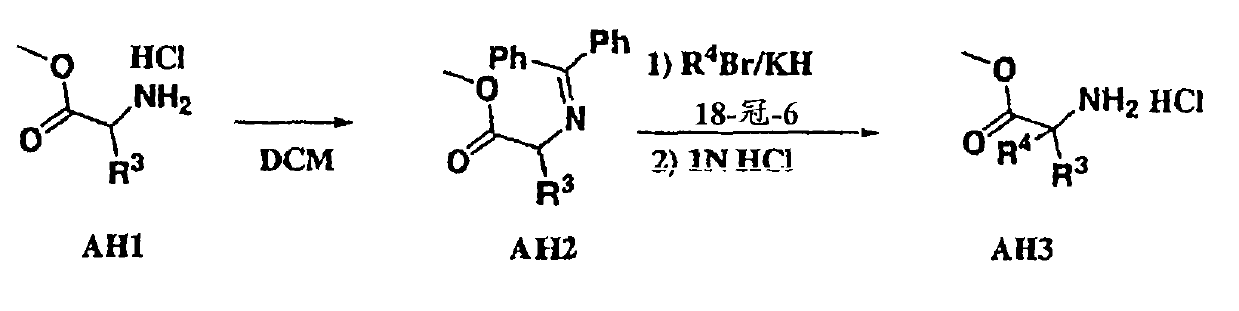
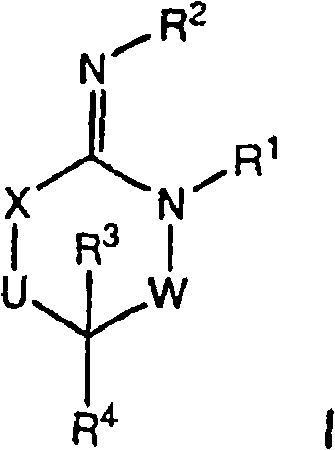
[0073] Compounds of formula I (wherein X, W and U are as defined above) include each of the following preferred structures:
[0074]
[0075] In compounds of formulas IA to IF, U is preferably a bond or -C(R 6 )(R 7 )-. In compounds of formula IG and IH, U is preferably -C(O)-.
[0076] It should be understood that since R 1 The definition of R 5 The definition of is the same, so when X is -N(R 5 )-, then W is a chemical bond and U is a chemical bond, -S(O)-, -S(O) 2 -, -C(O)-, -O-, -C(R 6 )(R 7 )-or-N(R 5 )-The compound of formula I is equivalent to U being a chemical bond and W being a chemical bond, -S(O)-, -S(O) 2 -, -C(O)-, -O-, -C(R 6 )(R 7 )-or-N(R 5 )- compound of formula I.
[0077] Compounds of the present invention are more preferably compounds of formula IB in which U is a chemical bond or U is -C(R 6 )(R 7 )- compound of formula IB.
[0078] Another preferred group of compounds of formula I are R 2 A compound of formula I which is H.
[0079] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com