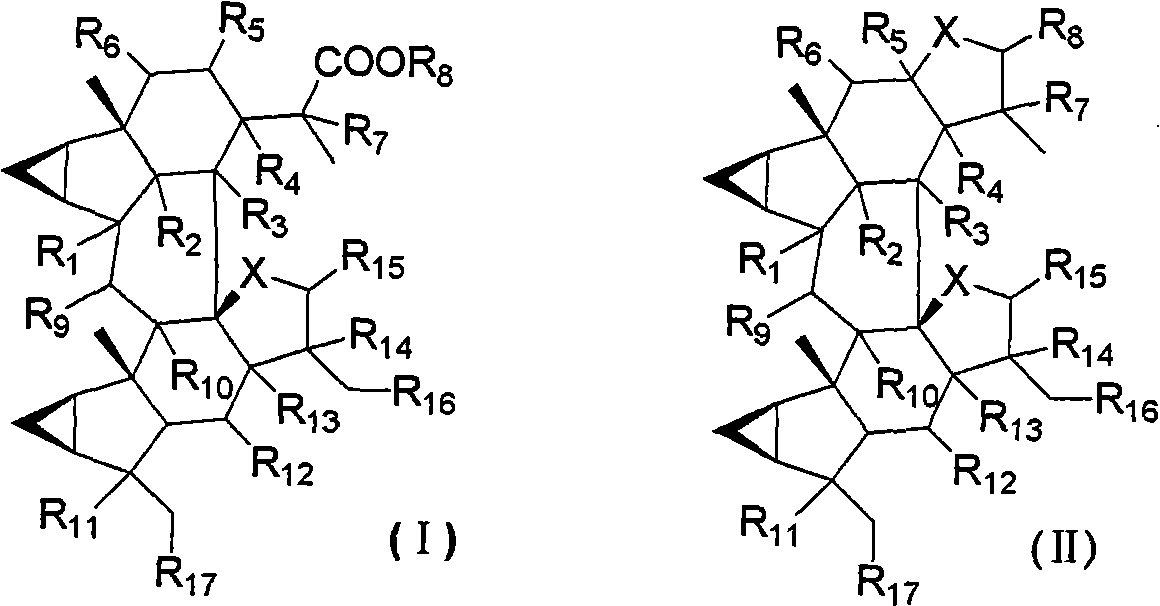
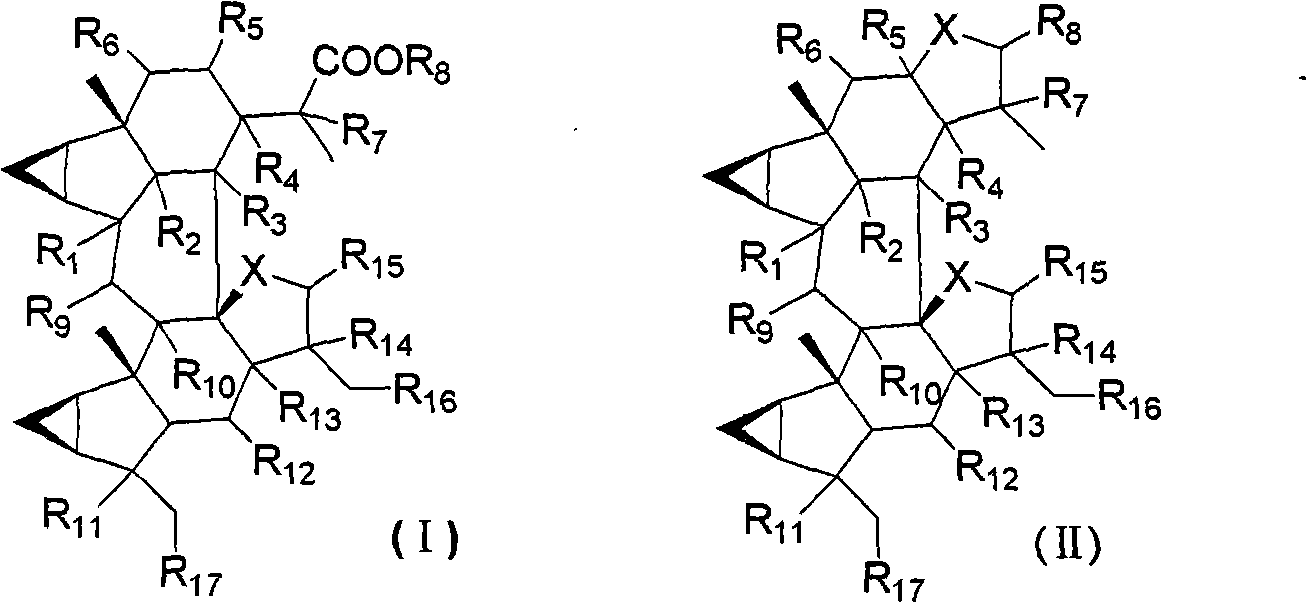
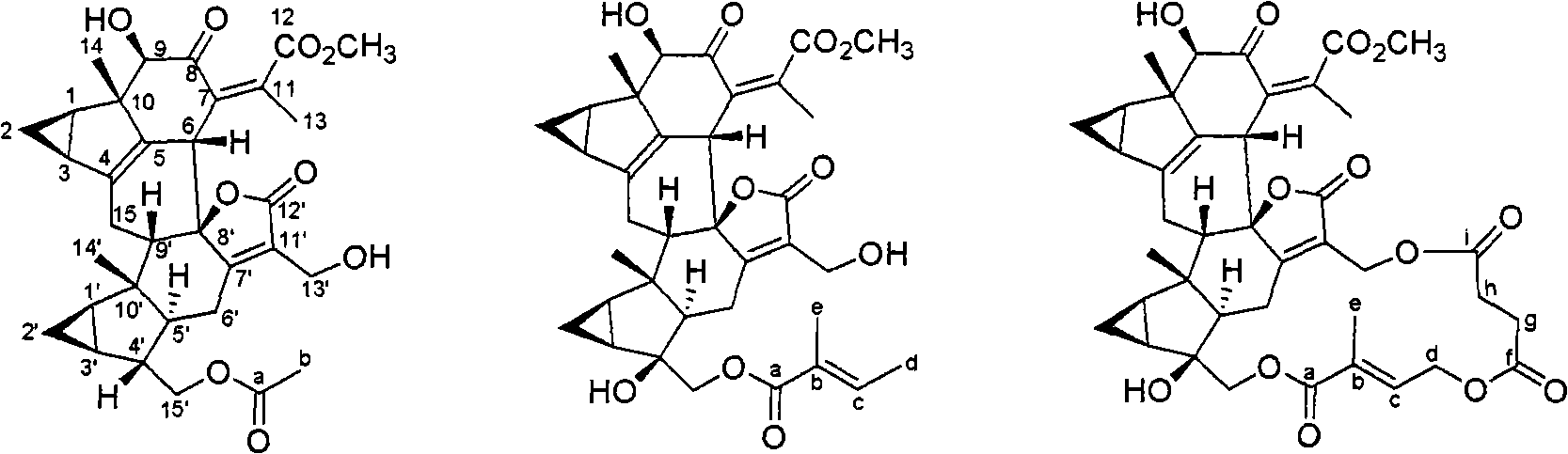
Lindenrane-type dimerization sesquiterpenoids, preparation method and applications thereof in pharmacy
A technology of terpenoids and uranine, which is applied to uranane-type dimerized sesquiterpenes and pharmaceutical compositions thereof, and the application field in the preparation of functional foods, and can solve adverse reactions, gastrointestinal discomfort, and no unpleasant symptoms. Urbolane-type dimerized sesquiterpenes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation of six compounds of silvergrass alcohol B-D, caprolactone A-B and chloramultilide C:
[0060] Chloranthus serratus (Chloranthus serratus) whole plant 8kg was pulverized, extracted with 70% acetone-water cooling for 3 times, each time for 48 hours, the extracts were combined, and the solvent was recovered under reduced pressure to obtain 200 g of extract. The extracted extract is dispersed in water, extracted three times with ethyl acetate and n-butanol successively, and the solvent is recovered to obtain ethyl acetate and n-butanol extract. Ethyl acetate extract (120g) was mixed with silica gel (80-100 mesh), followed by silica gel (200-300 mesh) column chromatography (eluent petroleum ether: acetone 10:1→1:2) gradient elution, TLC The detection was combined to obtain 6 fractions (Fr.1-6). Fr.4 and Fr.5 were subjected to Rp-18 reverse phase column chromatography (MeOH-H 2 O 3:7→7:3), Saphadex LH-20, normal phase silica gel column chromatography (CHCl 3 -M...
Embodiment 2
[0062] Preparation of ten compounds of silvergrass alcohol C-D, chloramultilide C-D and spicranol A-F:
[0063] Chloranthus multistachys (Chloranthus multistachys) whole plant 20kg crushed, reflux extracted with methanol 3 times, combined extracts, recovered solvent under reduced pressure to obtain extract. The extracted extract is dispersed in water, extracted three times with ethyl acetate and n-butanol successively, and the solvent is recovered to obtain ethyl acetate and n-butanol extract. Ethyl acetate extract (1300g) was passed through
[0064] MCI gel column chromatography (eluent 30% MeOH, 50% MeOH, 75% MeOH, 100% MeOH) obtains 4 parts, and 75% MeOH elution part is through silica gel column chromatography (eluent sherwood oil: acetone= 10:1 → 1:1) was divided into 4 parts, the third part was taken, after repeated Saphadex LH-20, normal phase silica gel column chromatography (CHCl 3 -MeOH 100:1 → 30:1), and HPLC reversed-phase semi-preparation, to obtain 10 buyalane-t...
Embodiment 3
[0094] According to the method of Example 1, six compounds of silverwort B-D, caprolactone A-B and chloramultilide C were first prepared, and water for injection was added as usual, finely filtered, potted and sterilized to make an injection.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap