A system capable of stably expressing cell cycle factor FoxM1 and its medical use
A cell cycle and expression vector technology, applied in the biological field, can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The construction of embodiment 1.FoxM1 non-viral vector
[0075] 1. Select the appropriate promoter
[0076] General promoters such as CMV, Caggs (CMV enhancer / chicken β-actin promoter / β-globin intron fusion sequence), liver-specific promoters such as albumin (Albumin), thyroxine binding protein ( TTR) promoters are optional promoters that regulate gene expression. In order to obtain efficient and stable expression of the FoxM1 gene, the inventors conducted a series of experiments to select the promoter.
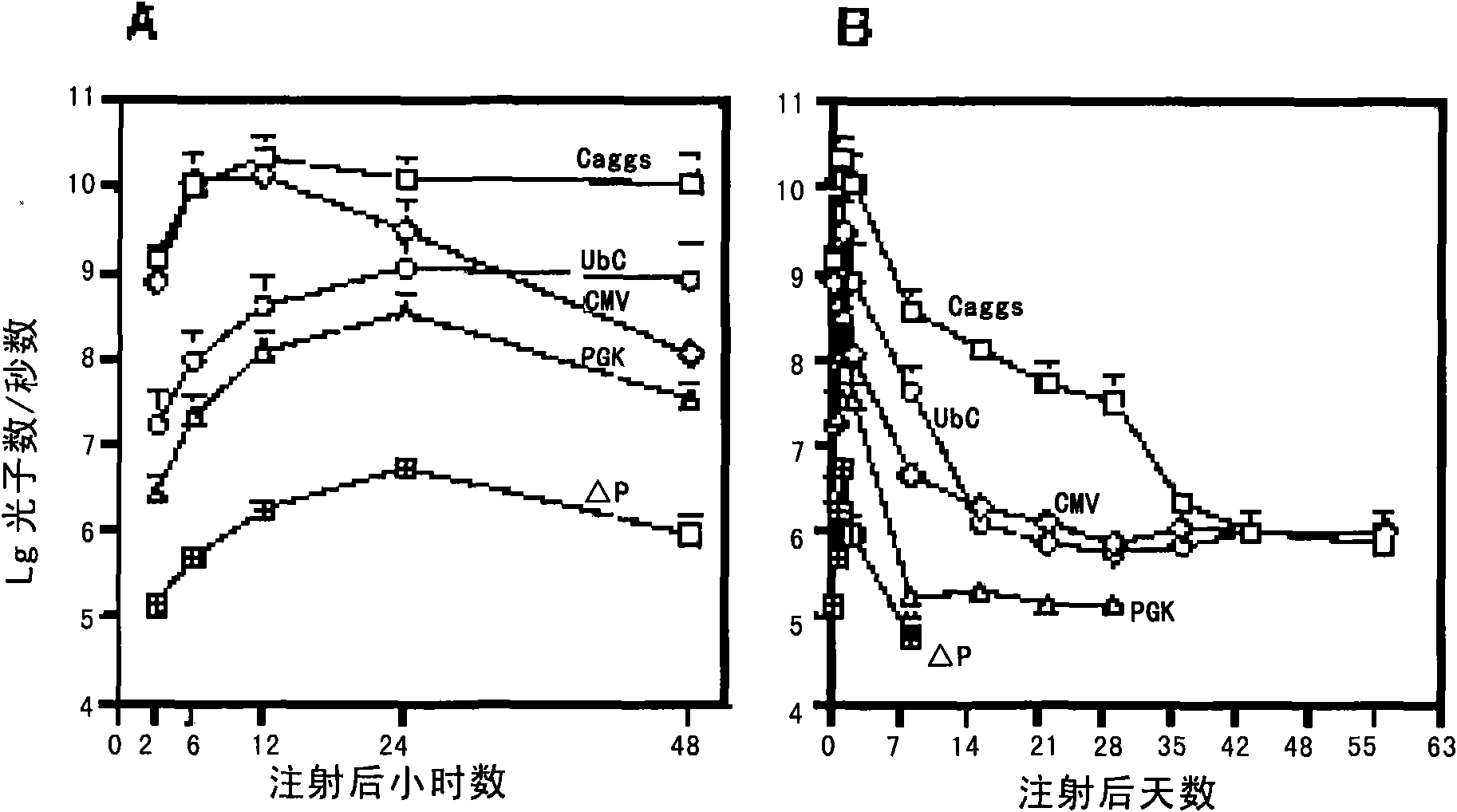
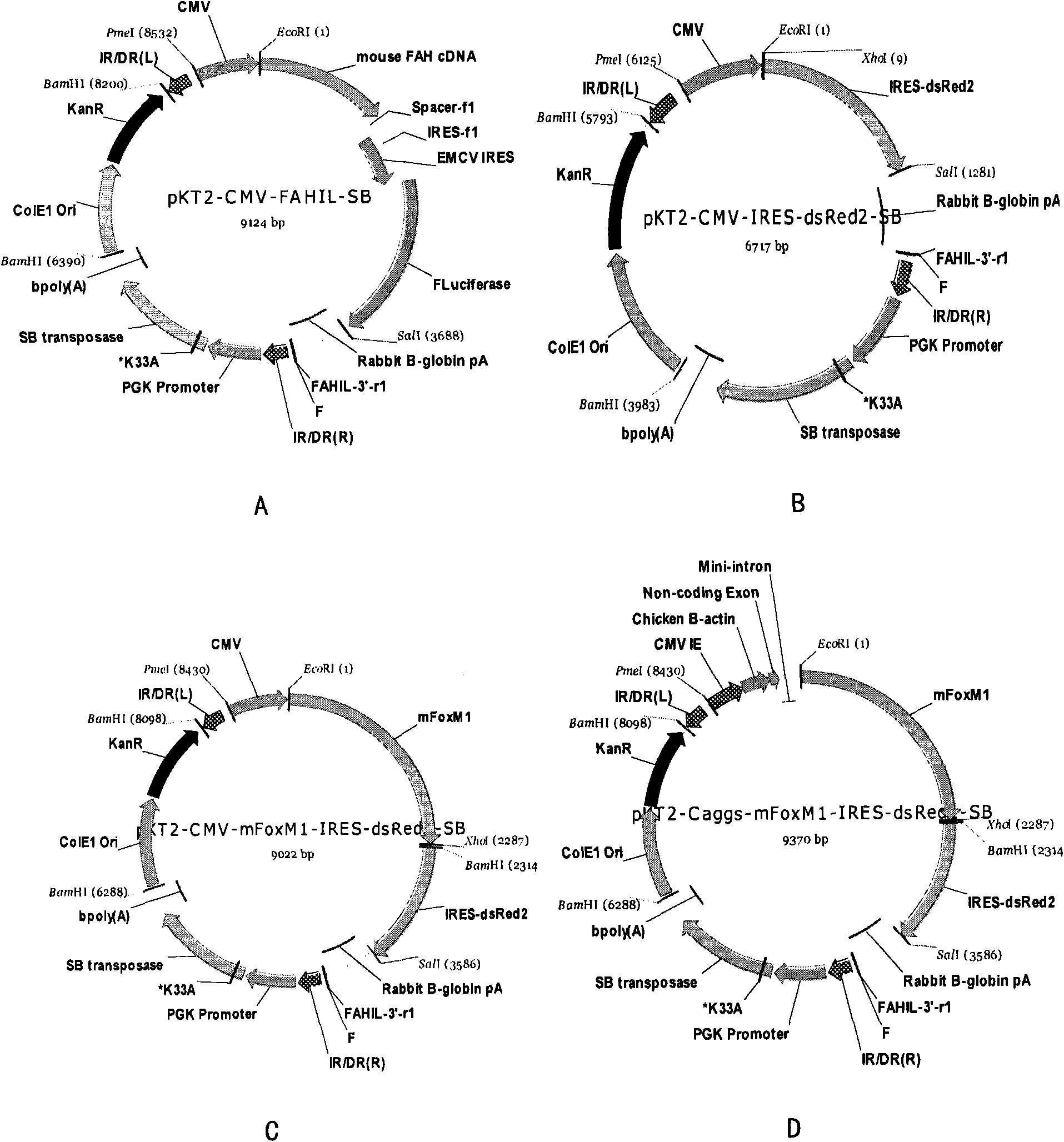
[0077] By detecting the differences in the expression levels of the luciferase gene driven by different promoters in the liver of the host, the inventors compared the differences of several promoters in liver gene therapy. Select different specific promoters and vector pKT2-CMV-FAHIL-SB ( figure 2 A, Obtained from Oregon Health and Science University; As the replacement of the CMV promoter in the expression vector 1), three other expression vectors were obtained, ...
Embodiment 2
[0085] Example 2. Obtaining hepatocytes stably expressing the FoxM1 gene
[0086] 1. In vitro transfection of liver cells with non-viral vector carrying FoxM1 gene
[0087] The present inventors isolated hepatocytes or mouse embryonic stem cells hepatically differentiated cells from adult livers of wild-type mice with a 129S4 background (obtained from Oregon Health and Science University) or fetal livers of 14 days of pregnancy, and digested the above cells into single Cell suspension, count 5×10 6 were inoculated on 10 cm culture dishes covered with type I collagen. The constructed pKT2-Caggs-mFoxM1-IRES-dsRed2-SB expression vector was used to transfect hepatocytes with Qiagen high-efficiency transfection reagent, and the transfected hepatocytes were screened by Ds-Red reporter gene and flow cytometry.
[0088] The work of the present inventors shows that the proportion of Ds-Red positive liver cells is between 5 and 10% through transposon transmission in cis. Ds-Red posit...
Embodiment 3
[0092] Example 3. Study on promoting liver repopulation after liver cell transplantation stably expressing FoxM1
[0093] The following mice were used as test materials:
[0094] Rosa-26β-gal transgenic mice: Obtained from Oregon Health and Science University, USA; the β-gal-labeled liver cells were used as control cells.
[0095] Immunodeficient Fah - / - mouse strain (Fah - / - Rag-2 - / - ): Obtained from Oregon Health and Science University, USA.
[0096] The following two evaluation systems for liver cell transplantation were selected:
[0097] Fah - / - Knockout mouse model: Wild-type hepatocytes also have a selective advantage in this model and can completely repopulate the liver. This mouse model was obtained from Oregon Health and Science University.
[0098] Wild-type mouse hepatectomy model: wild-type hepatocytes have no selective advantage in this model. The mouse model was purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences.
[0099] 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com