Pharmaceutical compositions for the treatment of chronic heart failure comprising pyrazolopyrimidinone derivative compound
A technology for chronic heart failure and pyrazolopyrimidone, which is applied in the field of pharmaceutical compositions for the treatment of chronic heart failure, can solve problems such as sudden swelling, and achieve the effects of reducing administration frequency, reducing inherent side effects, and increasing plasma levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
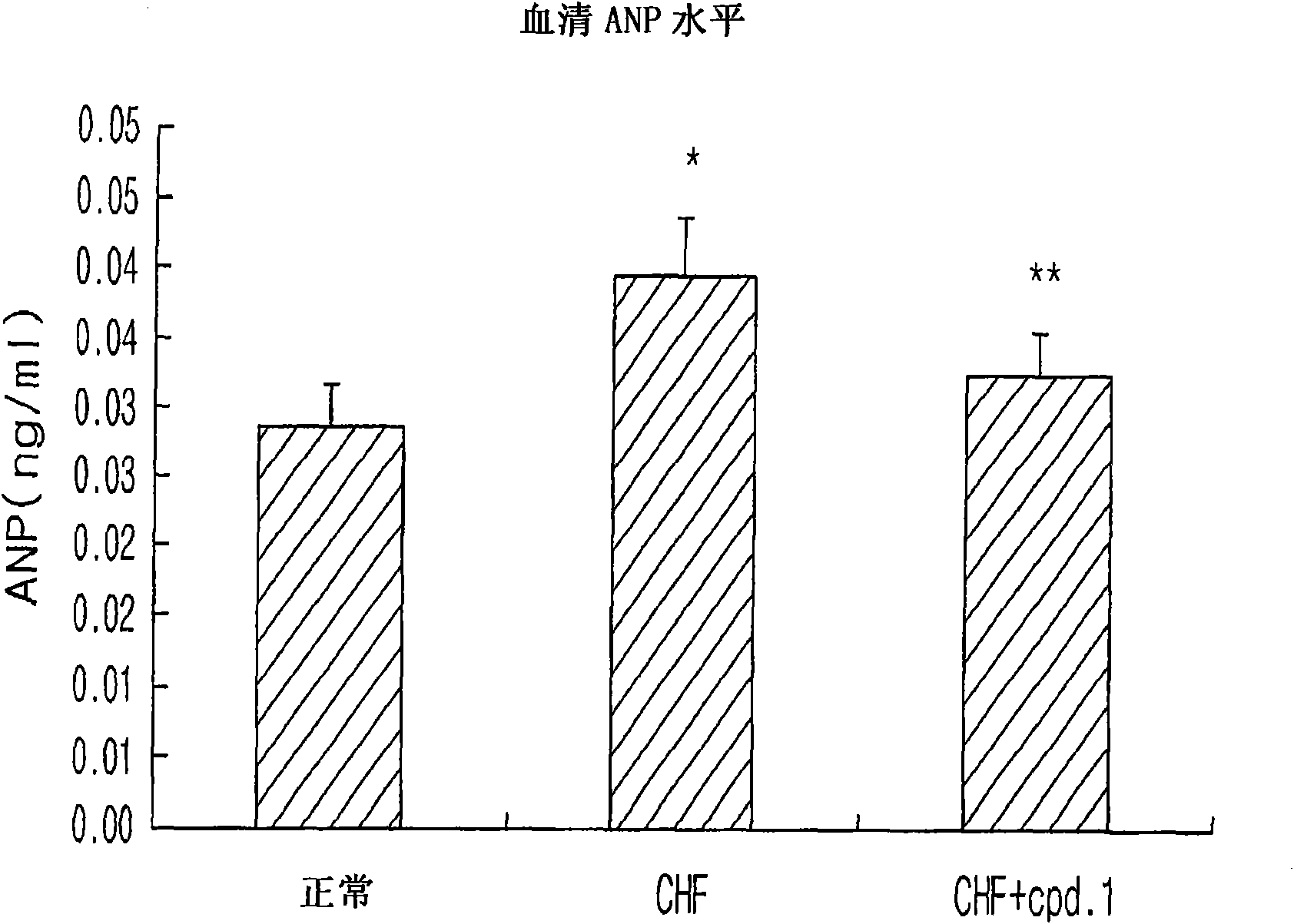
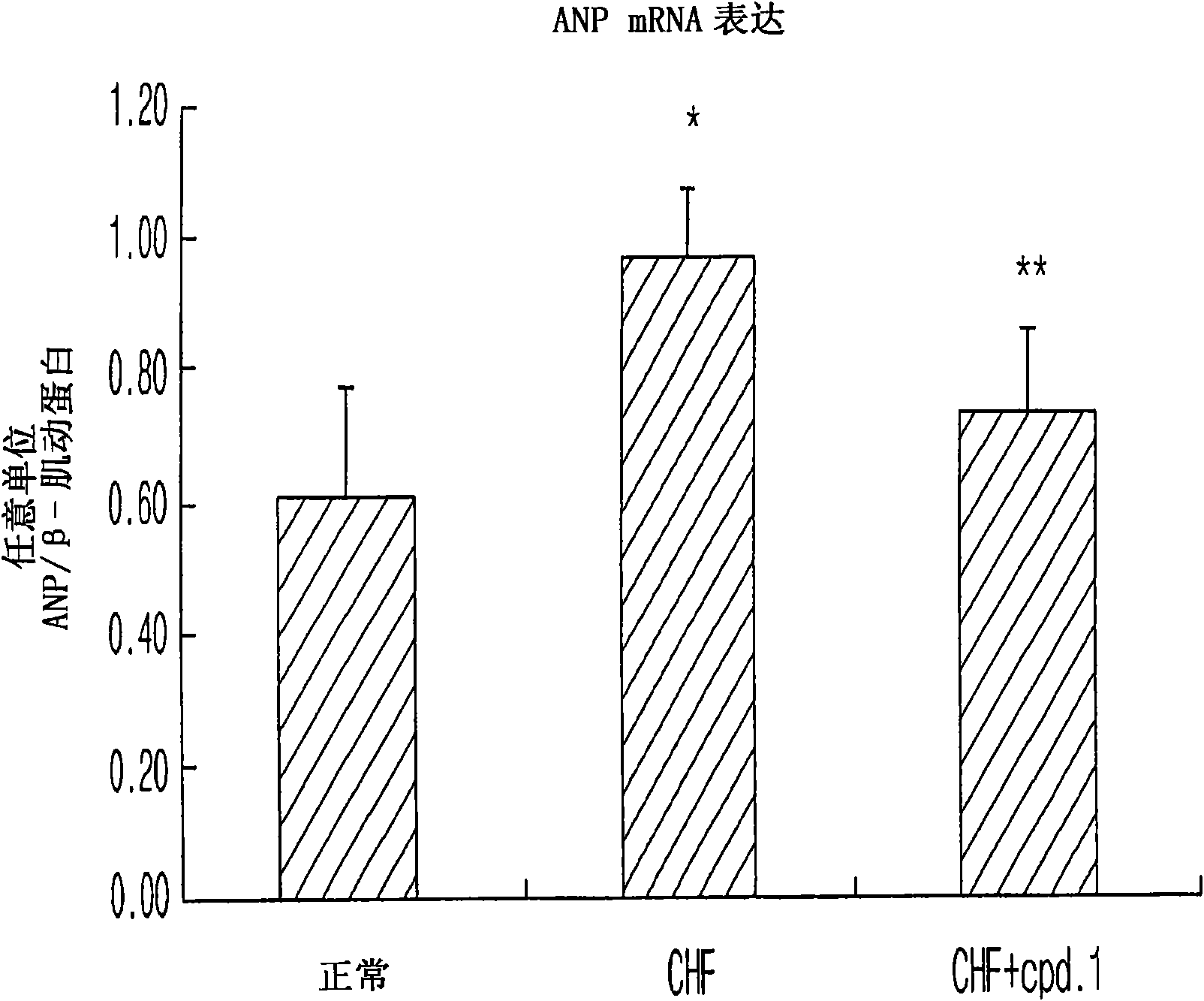
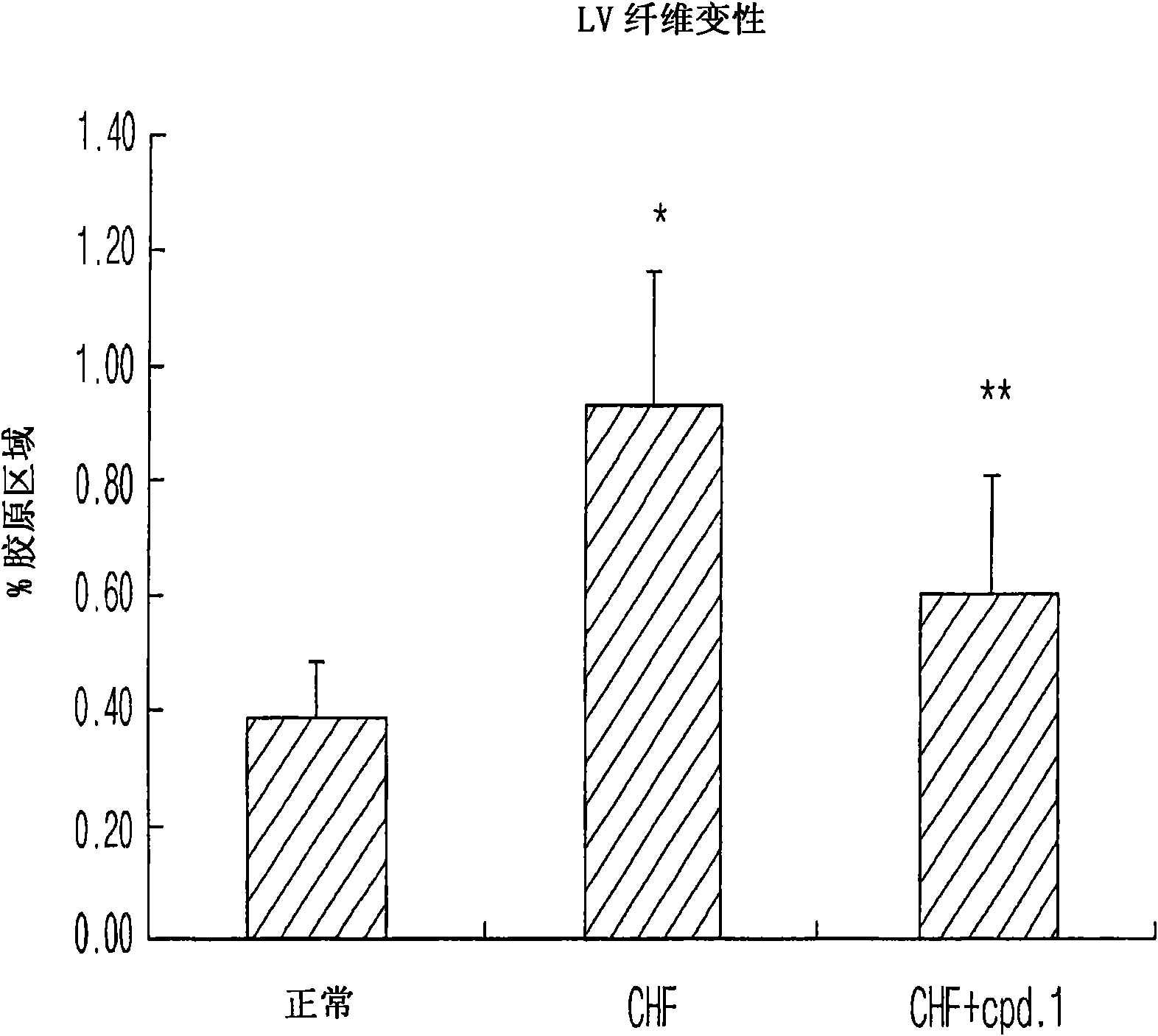
[0047] Example: Evaluation of the therapeutic effect of pyrazolopyrimidinone derivatives in animal models of heart failure
[0048] The pyrazolopyrimidinone derivative of Chemical Formula 1 according to the present invention was evaluated as follows to determine whether it has a therapeutic effect on heart failure.
[0049] Male Sprague-Dawley rats weighing 220-240 g were randomly divided into three groups: normal control group (normal), heart failure control group (CHF) and treatment group (CHF plus cpd.1), each consisting of seven large rat composition. The pyrazolopyrimidinone derivative was orally administered at a dose of 30 mg / kg to animals in the treatment group that had developed heart failure.
[0050] Normal control animals were sham-operated. Heart failure control and treatment animals underwent surgical abdominal incision to create an abdominal aorto-inferior vena cava fistula to artificially increase blood flow from the inferior vena cava to the right atrium. E...
preparation Embodiment
[0062] Formulation Example: Preparation of Pharmaceutical Formulations for Oral Administration
[0063] 1. Preparation of powder
[0064] Compound of formula 1 2 g
[0065] Lactose 1g
[0066] The above ingredients are mixed and placed in an airtight package to produce a powder.
[0067] 2. Preparation of tablets
[0068] Compound of formula 1 100mg
[0069] Corn starch 100mg
[0070] Lactose 100mg
[0071] Magnesium Stearate 2mg
[0072] The above ingredients are mixed and compressed according to conventional tablet manufacturing methods to produce tablets.
[0073] 3. Preparation of Capsules
[0074] Compound of formula 1 100mg
[0075] Corn starch 100mg
[0076] Lactose 100mg
[0077] Magnesium Stearate 2mg
[0078] The above-mentioned ingredients are mixed and loaded into gelatin capsules according to a conventional capsule manufacturing method to prepare capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com