Methods of treating RSV infections and related conditions
A technology of A4B4L1FR-S28R and A4B4-F52S, applied in chemical instruments and methods, antiviral agents, pharmaceutical formulations, etc., can solve problems such as impaired lung function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
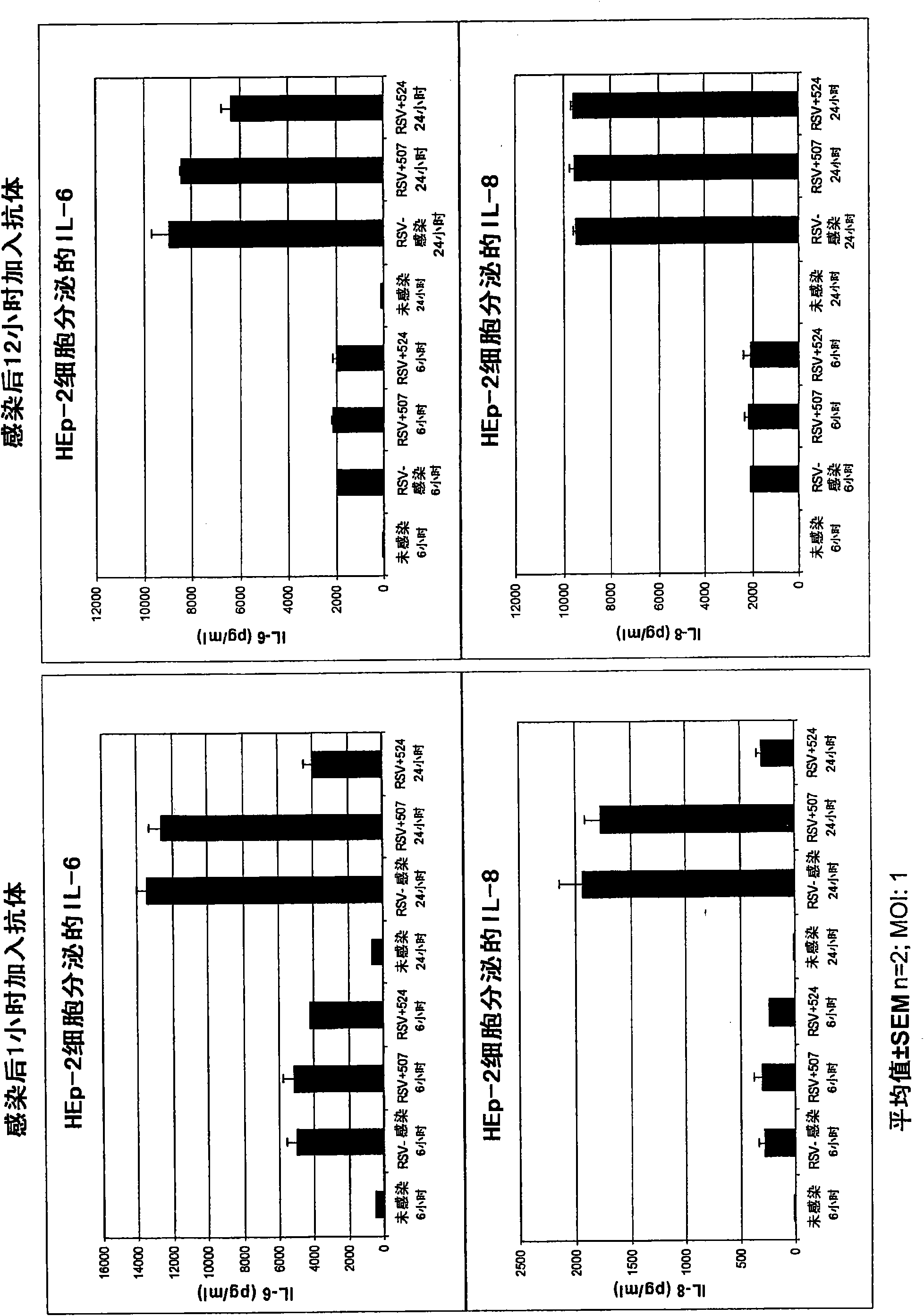
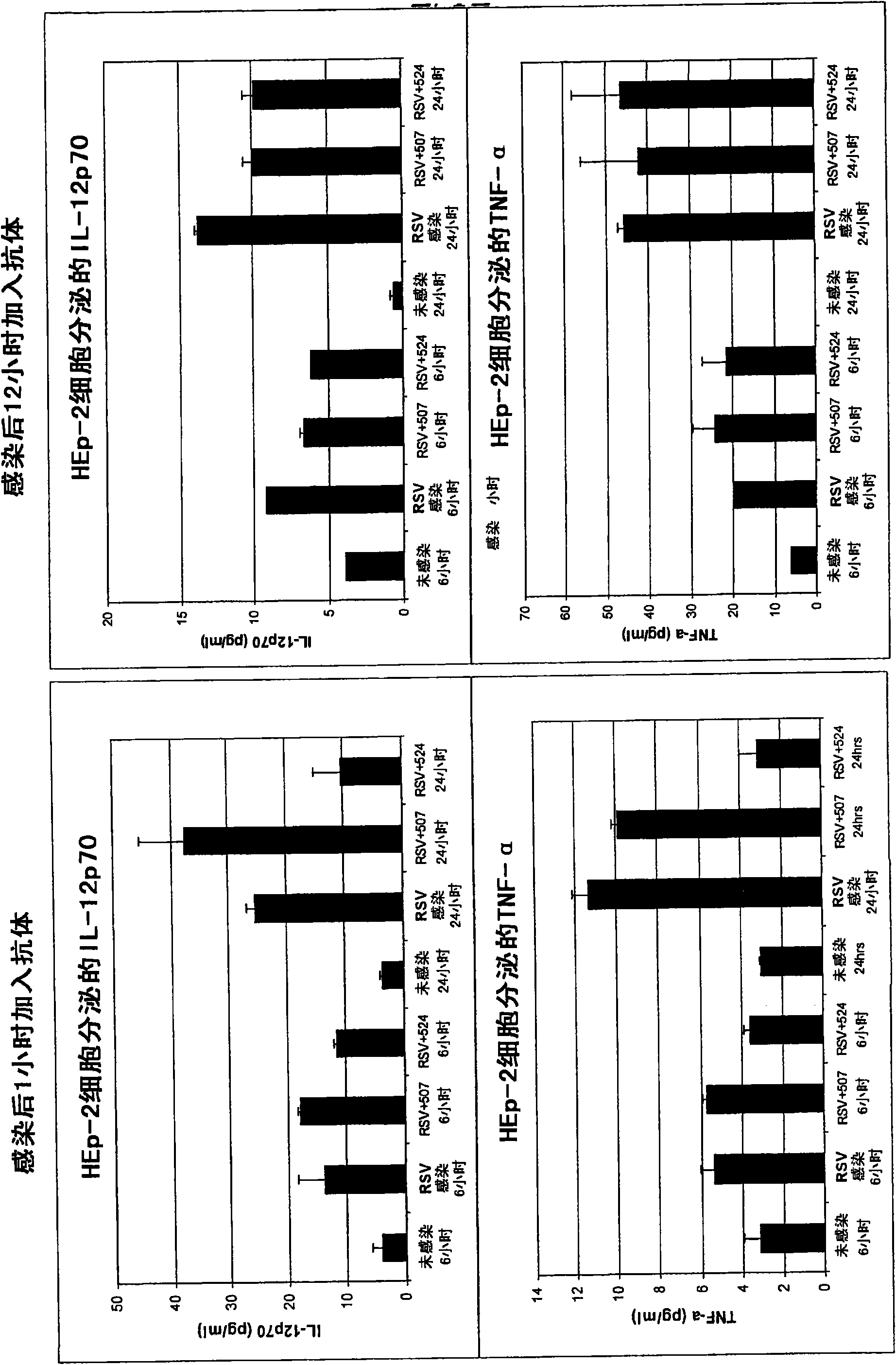
[0399] Example 1: MEDI-524 treatment modulates RSV-induced cytokine response
[0400] After infection, MEDI-524 was added to RSV-infected epithelial cells to observe whether the administration of antibodies can regulate the release of cytokines from RSV-infected cells. The test was conducted at two infection time points, one was 1 hour after infection and the other was 12 hours after infection.
[0401] 12 hours time point: Inoculate 2ml HEp-2 (9th passage) cells in 2-12 wells at a density of 5x10 5 Cells / well, culture for about 1 day to confluence. The confluent Hep-2 cells were infected with RSV A virus (WVB032302) MOI=1. After 12 hours of infection, control antibody MEDI-507 (20ug / ml) or MEDI-524 (52405G-0964) (20ug / ml) was added to the appropriate wells. Then at 37℃ / 5%CO 2 Incubate the cells for 6 and 24 hours. Centrifuge at 1500 rpm for 5 minutes at 6 or 24 hours to collect the supernatant and store it at -80°C until use.
[0402] 1 hour time point: Inoculate 2ml HEp-2P10 c...
Embodiment 2
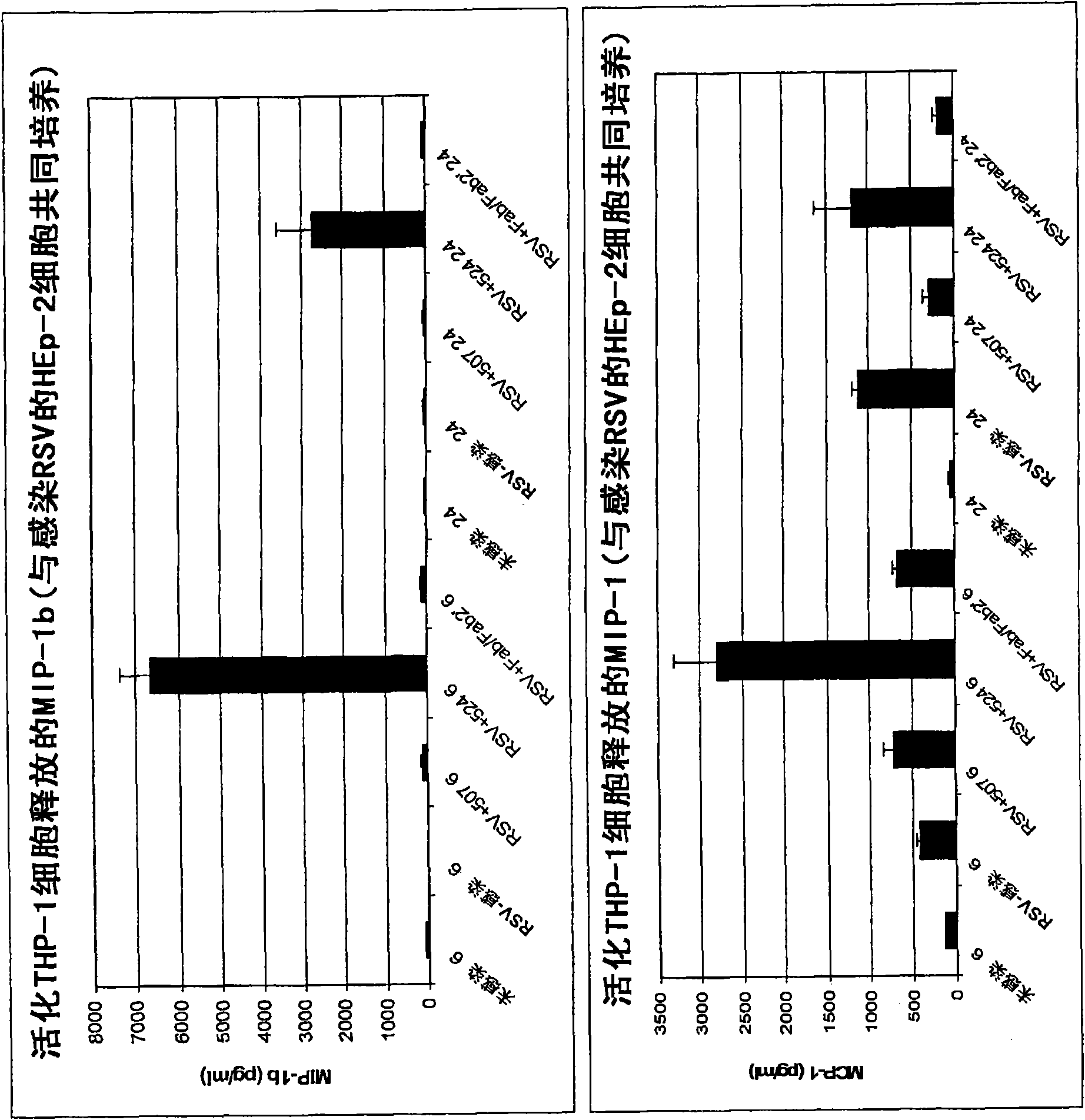
[0404] Example 2: MEDI-524-mediated THP-1 activation
[0405] Experiments were performed to determine whether MEDI-524 treatment can modulate the chemokine response of activated THP-1 cells in response to RSV-infected cells.
[0406] Inoculate 2ml HEp-2 cells (passage 8) in 4-12 wells at a density of 5x10 5 Cells / well. Use IFN-γ (500U / ml final concentration, 15 μl for 15 ml) to activate the 14th generation (3x10 5 Cells / ml, 15ml) and the 27th passage (3.0x10 5 Cells / ml, 15ml) THP-1 cells for 48 hours.
[0407] After culturing for about 36 hours, use RSV A (8x10 6 pfu / ml) HEp-2 cells confluent in 12 wells were infected with MOI=1. After 12-15 hours, aspirate the infection medium and rinse once with FACS buffer (1xPBS containing 2% FBS). Dilute the control antibodies MEDI-507, MEDI-524 (52405G-0336, 10.2mg / ml) or MEDI-524Fab'2 (KS011107, 2.75mg / ml) with FACS buffer to a final concentration of 20ug / ml, and add them to each well . After incubating for 15 minutes at room temperature,...
Embodiment 3
[0410] Example 3: THP-1 phagocytosis mediated by MEDI-524
[0411] Experiments were performed to determine whether MEDI-524 treatment can mediate the phagocytosis of RSV-infected cells by monocytes.
[0412] Stain HEp-2 cells with lipophilic dyes: count the 5th generation HEp-2 cells at 1x10 6 Cells / ml are resuspended in HBSS in a 50ml conical tube. Next, add 2.5ul blue dye per ml of HBSS cell suspension ( DiD cell labeling solution, #V22887, Yingjie The cells were incubated at 37°C for 20 minutes, and the 50 ml conical tube was inverted 3 times every 5 minutes. The cells were washed 4 times with HBSS at 1700 rpm for 5 minutes. Resuspend the cells in complete medium and seed them in a 12-well plate with a seeding density of 5x10 5 Cells / well, seeding volume 2 ml (cells are confluent within 48 hours).
[0413] Activation of THP-1 cells: Use 500U / ml IFN-γ (12ul, for 12ml THP-1 cells) to activate the 19th generation of THP-1 cells (3x10 5 Cells / ml, 12ml), incubated at 37°C for 48 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com