A kind of dexketoprofen coated sustained-release microparticle capsule
A technology of dexketoprofen and coating sustained-release, which is applied in the directions of non-active ingredients medical preparations, organic active ingredients, non-central pain relievers, etc., and can solve the problems of poor drug release reproducibility and large individual differences. , to achieve the effect of improving bioavailability, eliminating irritation and improving therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
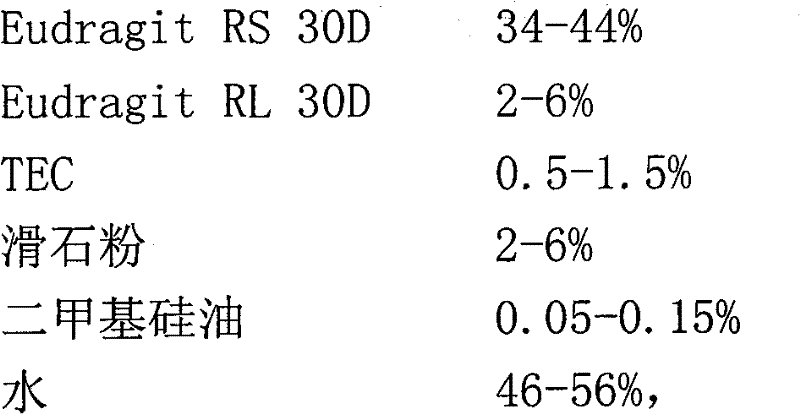
Image
Examples
Embodiment 1
[0117] Core Particle Formula:
[0118] weight
weight percentage
Dexketoprofen
682g
68.2%
MCC
91g
9.1%
lactose
136g
13.6%
6% HPMC solution (6cps)
91g
9.1%
common
1000g
100%
[0119] Core particle preparation process:
[0120] 1. Weigh HPMC (6cps), add it to an appropriate amount of water under stirring, and stir until clarified to make a 6% aqueous solution for later use;
[0121] 2. Weigh the dexketoprofen, lactose, and MCC of the formula, pass through an 80-mesh sieve respectively, and mix thoroughly;
[0122]3. Put the uniformly mixed auxiliary materials into the fluidized bed of the multifunctional granulation coating machine (powder coating machine), and check the tightness of the fluidized bed;
[0123] 4. Adjust the air volume to keep the material in a fluidized state. The air volume (0-15min) at the initial stage of granulation is 20-25m 3 / h, continuous ...
Embodiment 2
[0140] Core Particle Formula:
[0141] weight
weight percentage
Dexketoprofen
730g
73%
MCC
60g
6%
lactose
100g
10%
6% HPMC solution (6cps)
110g
11%
common
1000g
100%
[0142] Core particle preparation process:
[0143] 1. Weigh HPMC (6cps), add it to an appropriate amount of water under stirring, and stir until clarified to make a 6% aqueous solution for later use;
[0144] 2. Weigh the dexketoprofen, lactose, and MCC of the formula, pass through an 80-mesh sieve respectively, and mix thoroughly;
[0145] 3. Put the uniformly mixed auxiliary materials into the fluidized bed of the multifunctional granulation coating machine (powder coating machine), and check the tightness of the fluidized bed;
[0146] 4. Adjust the air volume to keep the material in a fluidized state. The air volume (0-15min) at the initial stage of granulation is 20-25m 3 / h, continuous air v...
Embodiment 3
[0163] Core Particle Formula:
[0164] weight
weight percentage
Dexketoprofen
630g
63%
MCC
120g
12%
lactose
160g
16%
6% HPMC solution (6cps)
90g
9%
common
1000g
100%
[0165] Core particle preparation process:
[0166] 1. Weigh HPMC (6cps), add it to an appropriate amount of water under stirring, and stir until clarified to make a 6% aqueous solution for later use;
[0167] 2. Weigh the dexketoprofen, lactose, and MCC of the formula, pass through an 80-mesh sieve respectively, and mix thoroughly;
[0168] 3. Put the uniformly mixed auxiliary materials into the fluidized bed of the multifunctional granulation coating machine (powder coating machine), and check the tightness of the fluidized bed;
[0169] 4. Adjust the air volume to keep the material in a fluidized state. The air volume (0-15min) at the initial stage of granulation is 20-25m 3 / h, continuou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com