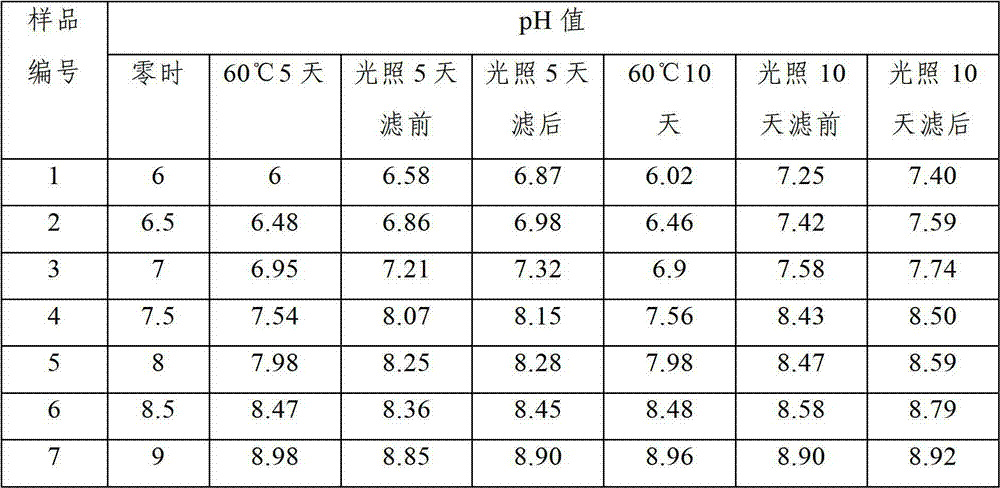
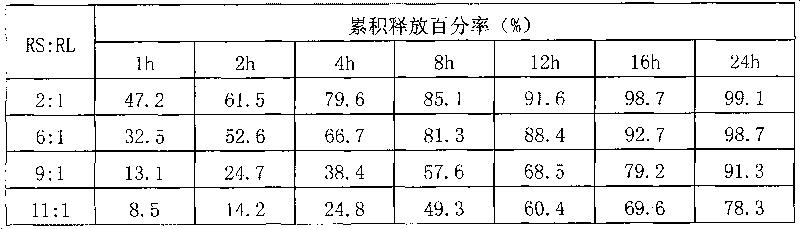
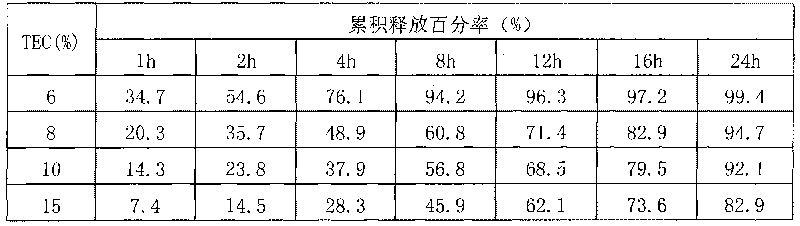
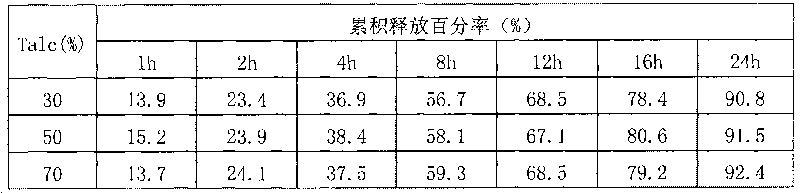
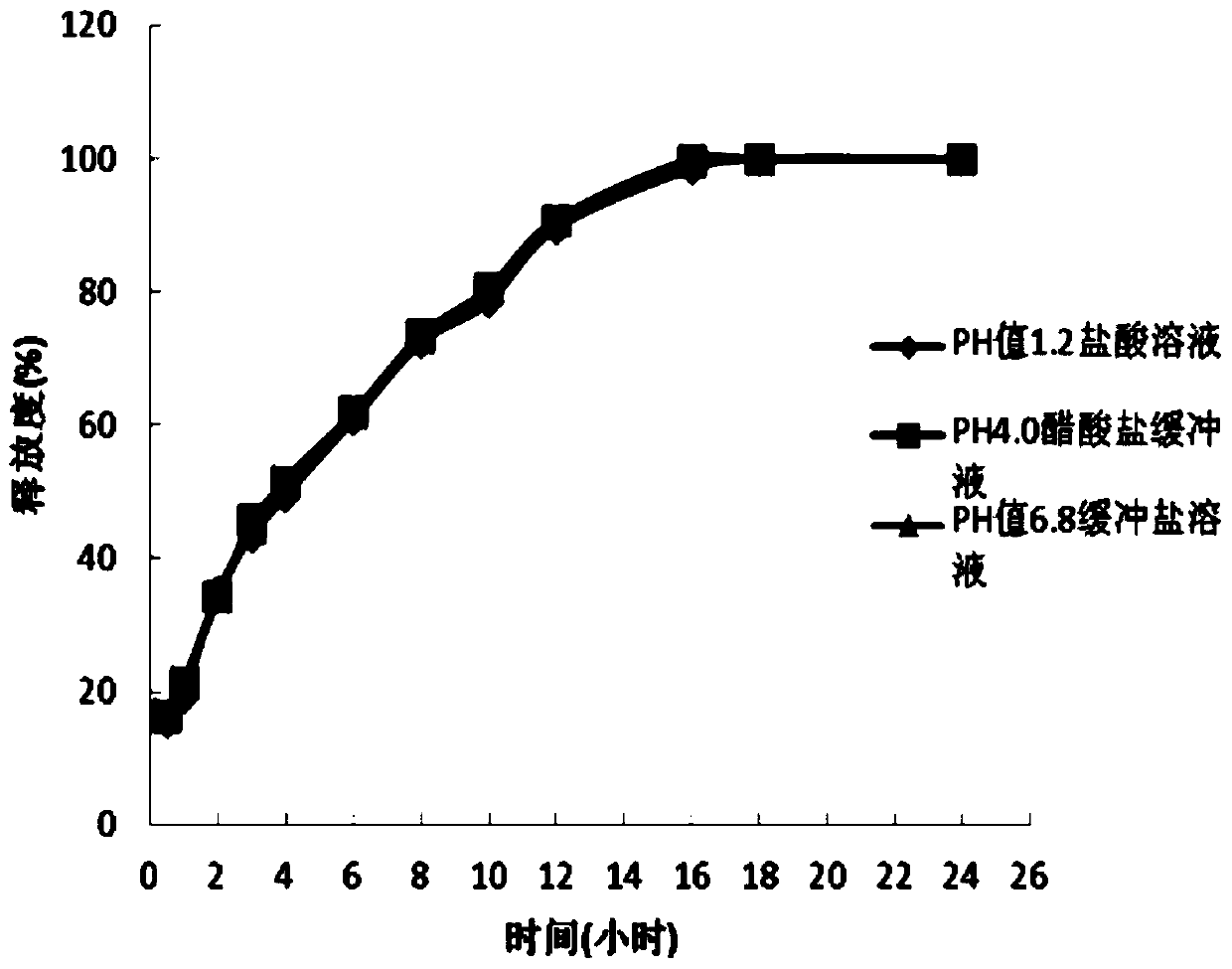
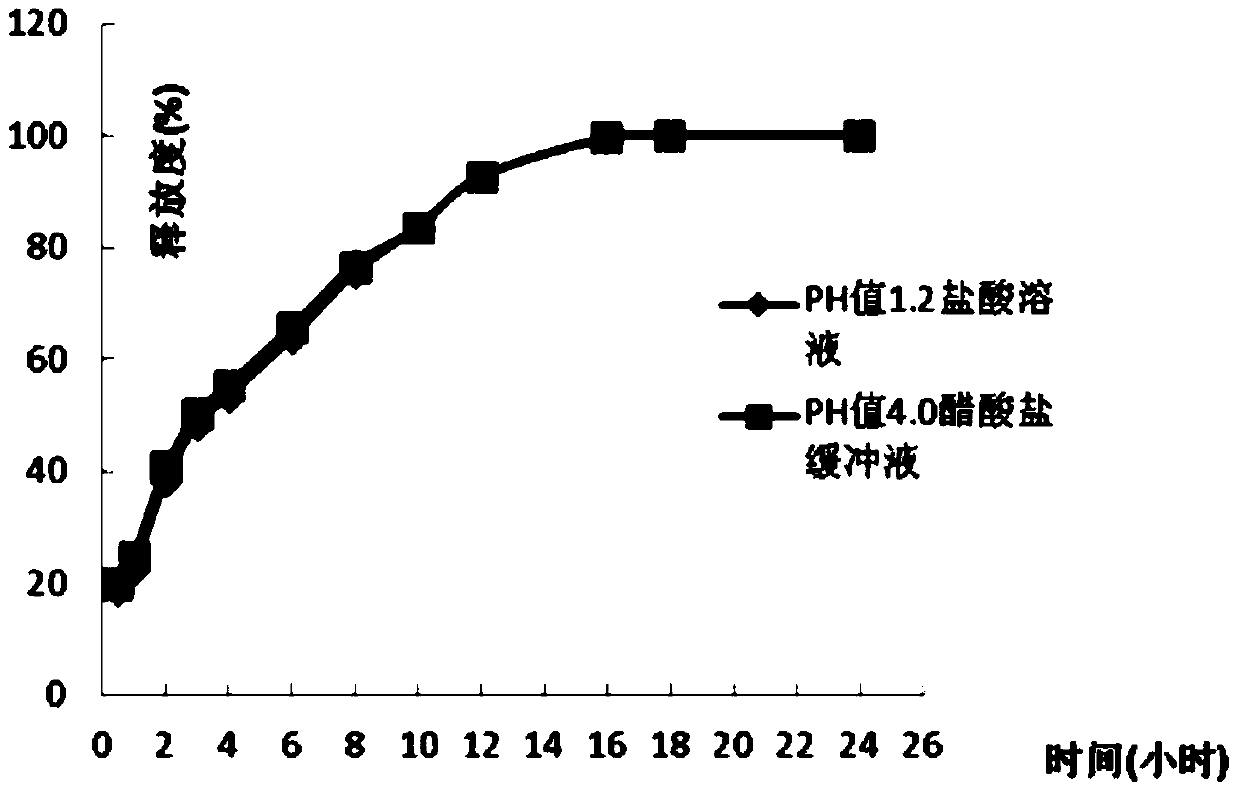
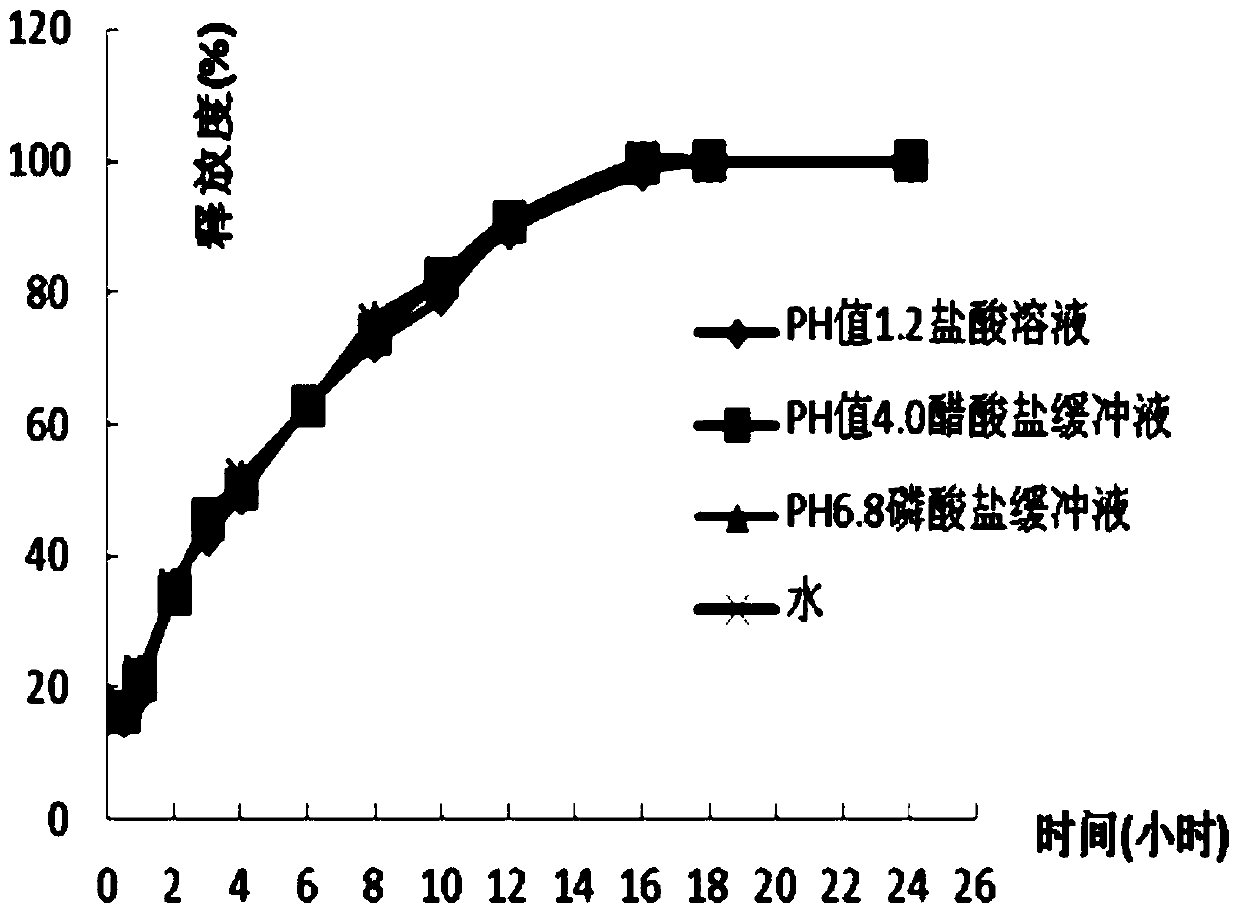
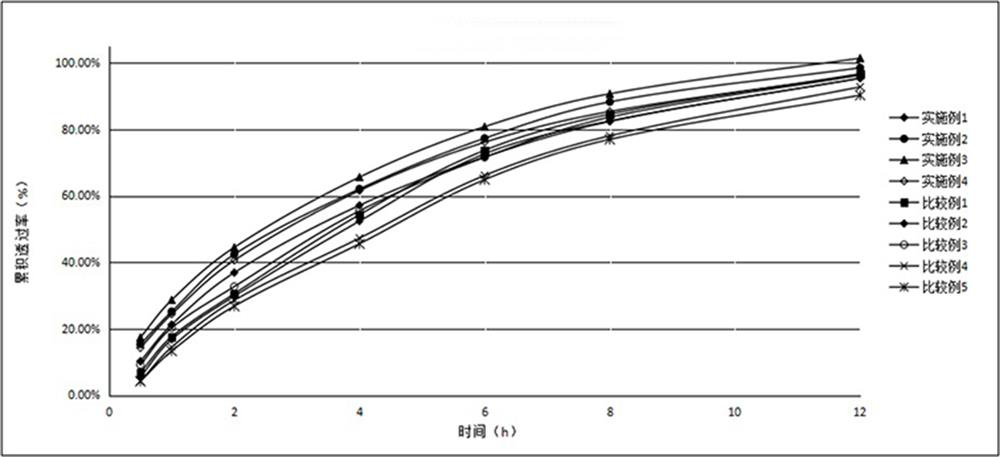
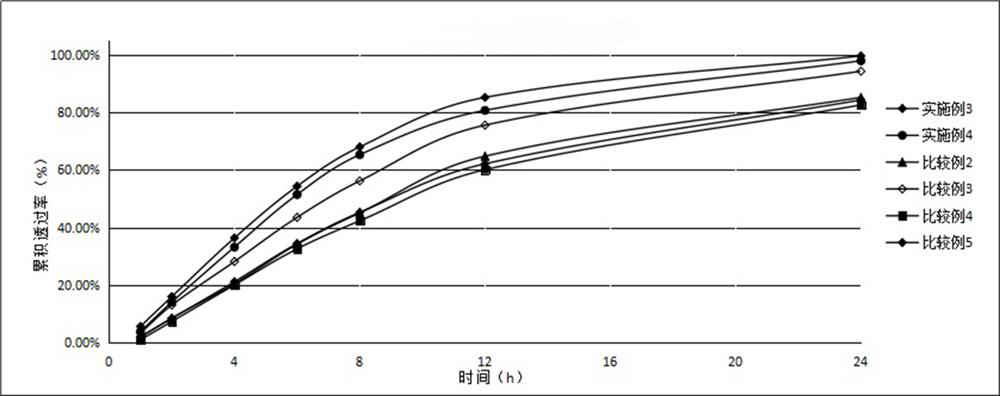
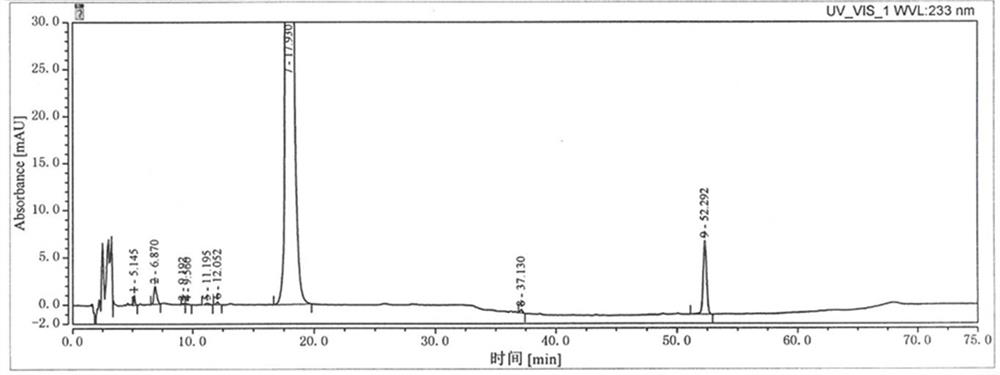
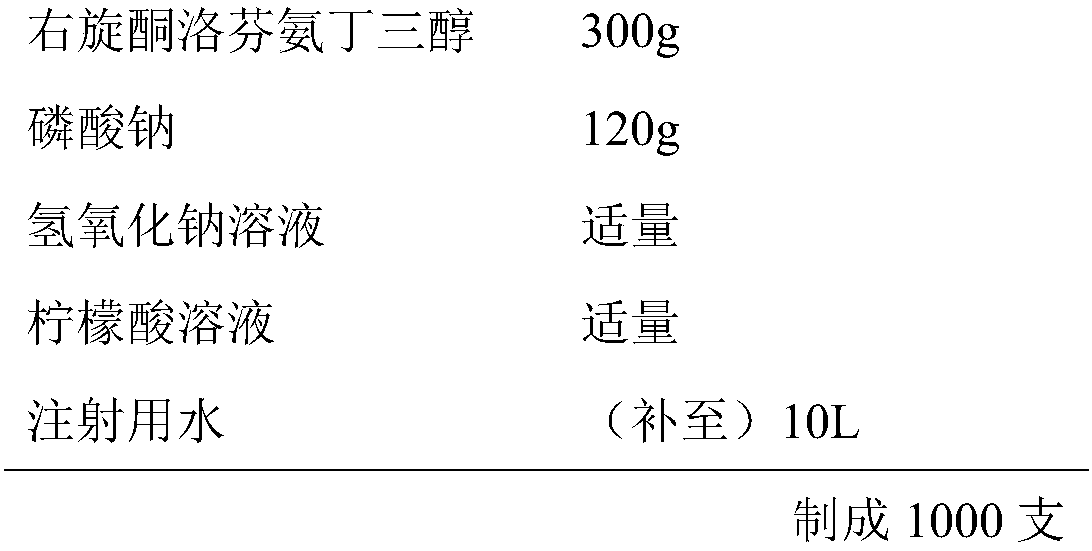
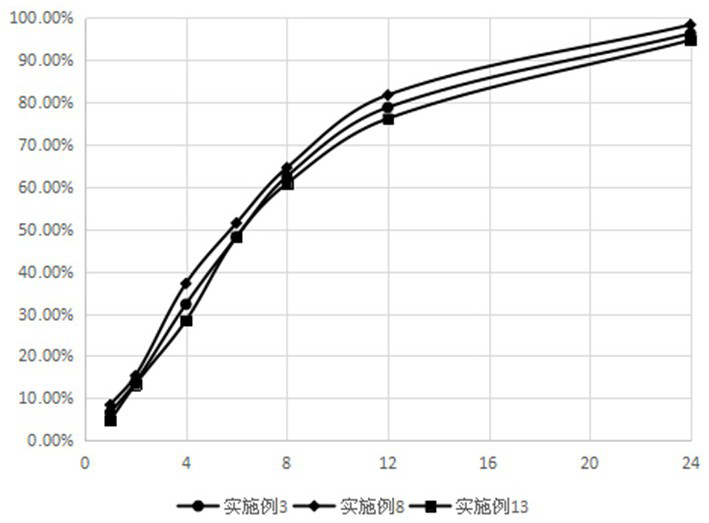
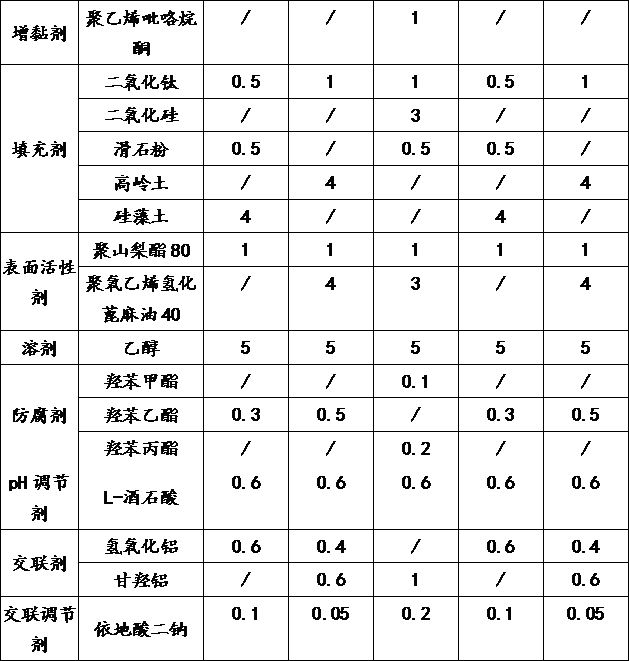
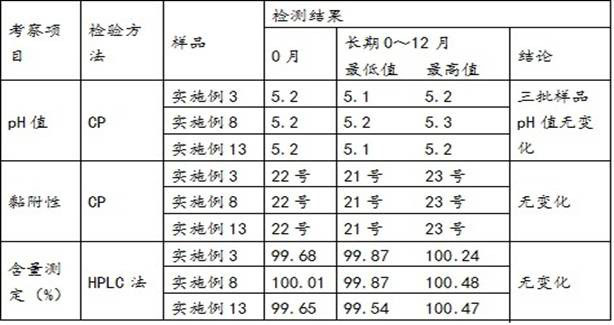
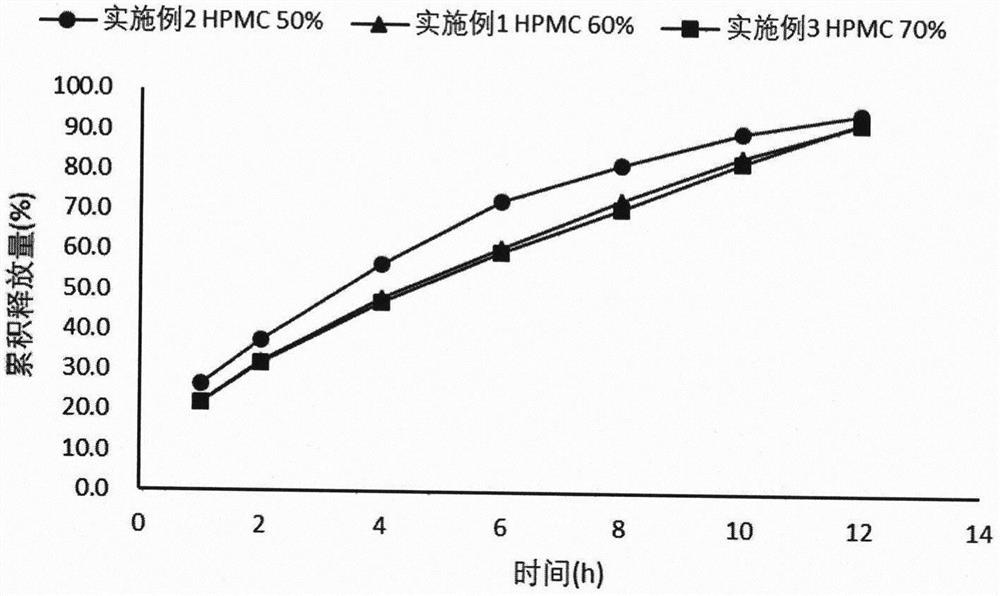
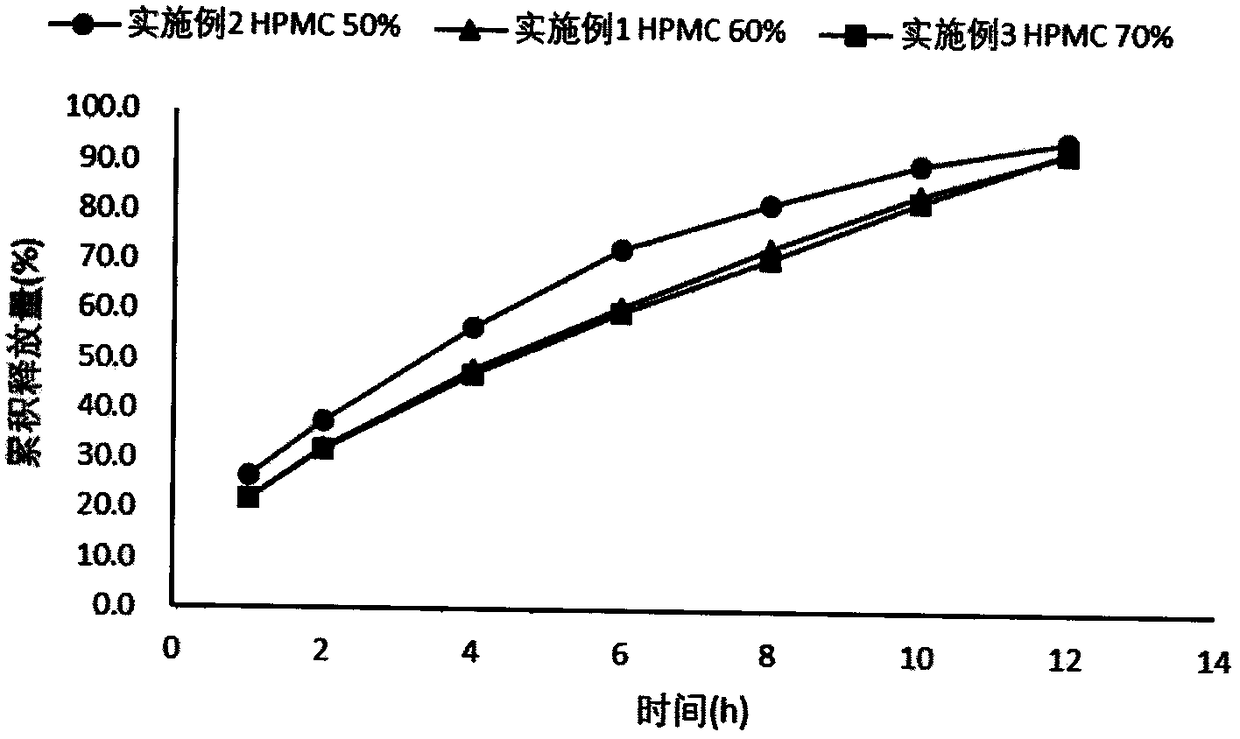
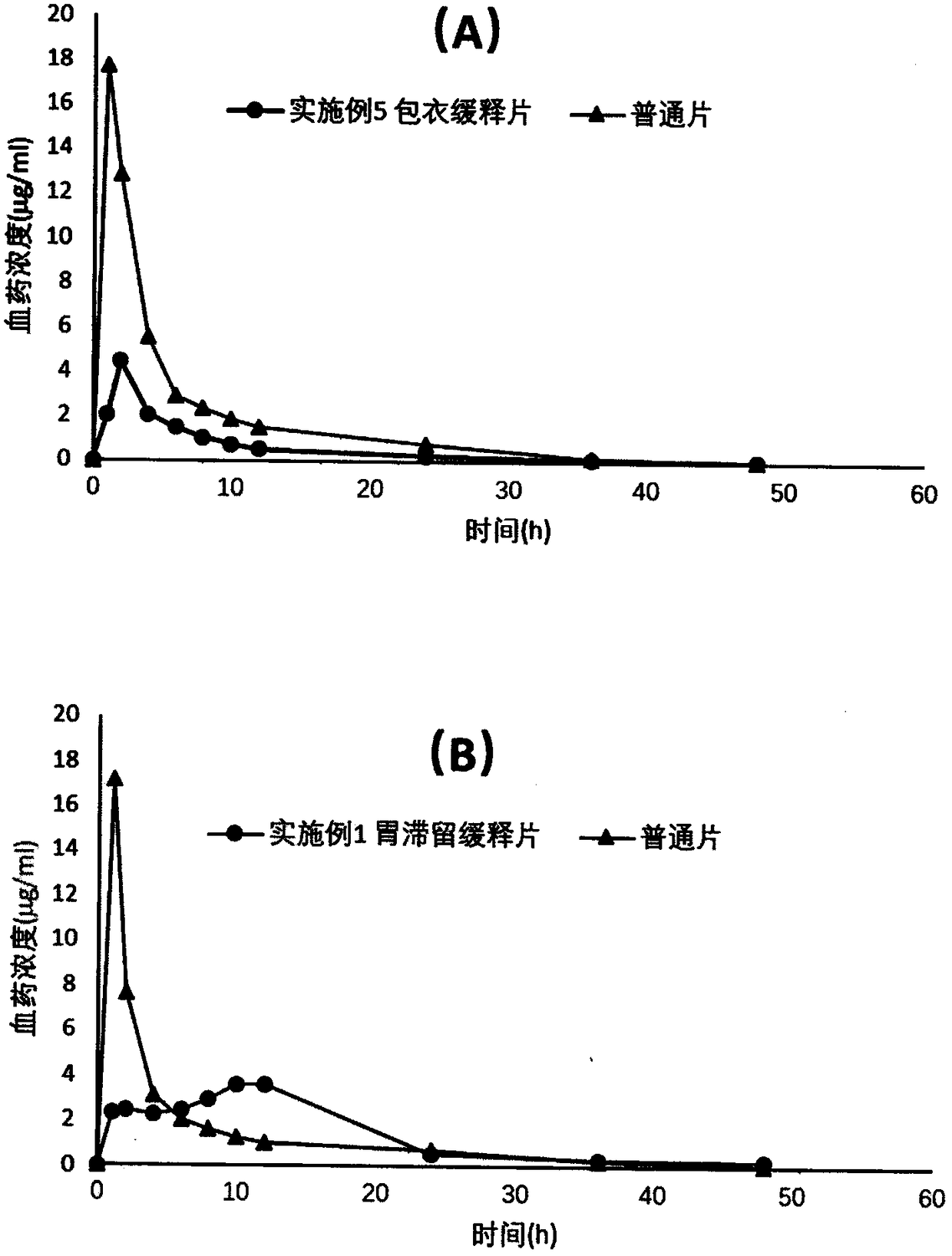
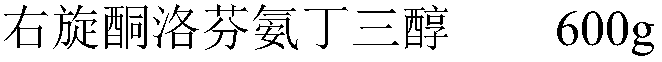Patents
Literature
32 results about "Dexketoprofen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
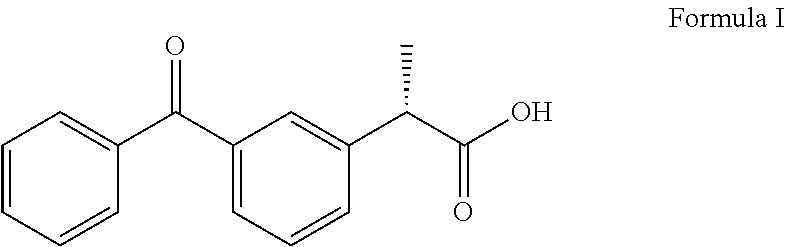
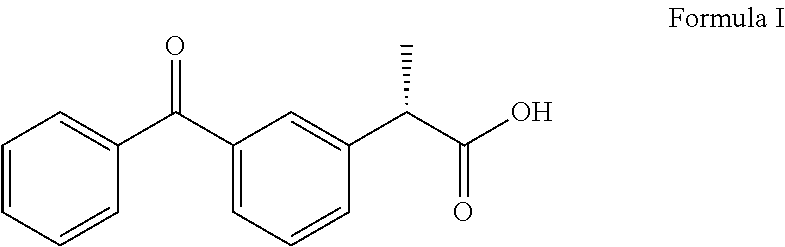
Dexketoprofen is a nonsteroidal anti-inflammatory drug (NSAID). It is manufactured by Menarini, under the tradename Keral. It is available in the UK, as dexketoprofen trometamol, as a prescription-only drug and in Latin America as Enantyum, produced by Menarini. Also, in Italy and Spain it is available as an over-the-counter drug (OTC) under the trade name Enantyum. In Latvia, Lithuania and Estonia it is available as an OTC under the tradename Dolmen In Mexico it is available in tablet form as "Stadium" made by Menarini. It is the dextrorotatory stereoisomer of ketoprofen.
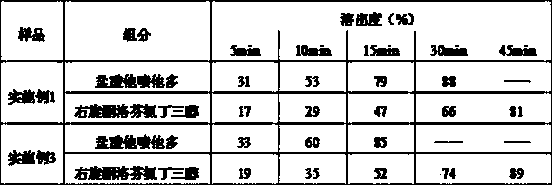
Gel type dexketoprofen plaster and method for preparing the same
InactiveCN1827094AIncrease local concentrationImprove complianceOrganic active ingredientsAntipyreticCross-linkMedicine
The invention discloses a gel type dexketoprofen plaster and method for preparation, which comprises backing material, gel type medicament material and anti-sticking material, wherein the medicament material contains 0.5-30.0 wt% of active medicinal constituents, 0.5-40.0 wt% of medicinal solvent, 0.5-30.0 wt% of humectant, and 0.5-8.0 wt% of transderrnal absorption promoting agent, the gel type medicinal material includes high- and low-molecular-weight poly vinyl pyrrolidone or copolymer containing vinyl pyrrolidone unit, polyvinyl alcohol, or polyvinyl alcohol and polyethylene glycol, cross linking agent and water. The invention also discloses its preparation.
Owner:HUAZHONG UNIV OF SCI & TECH +1
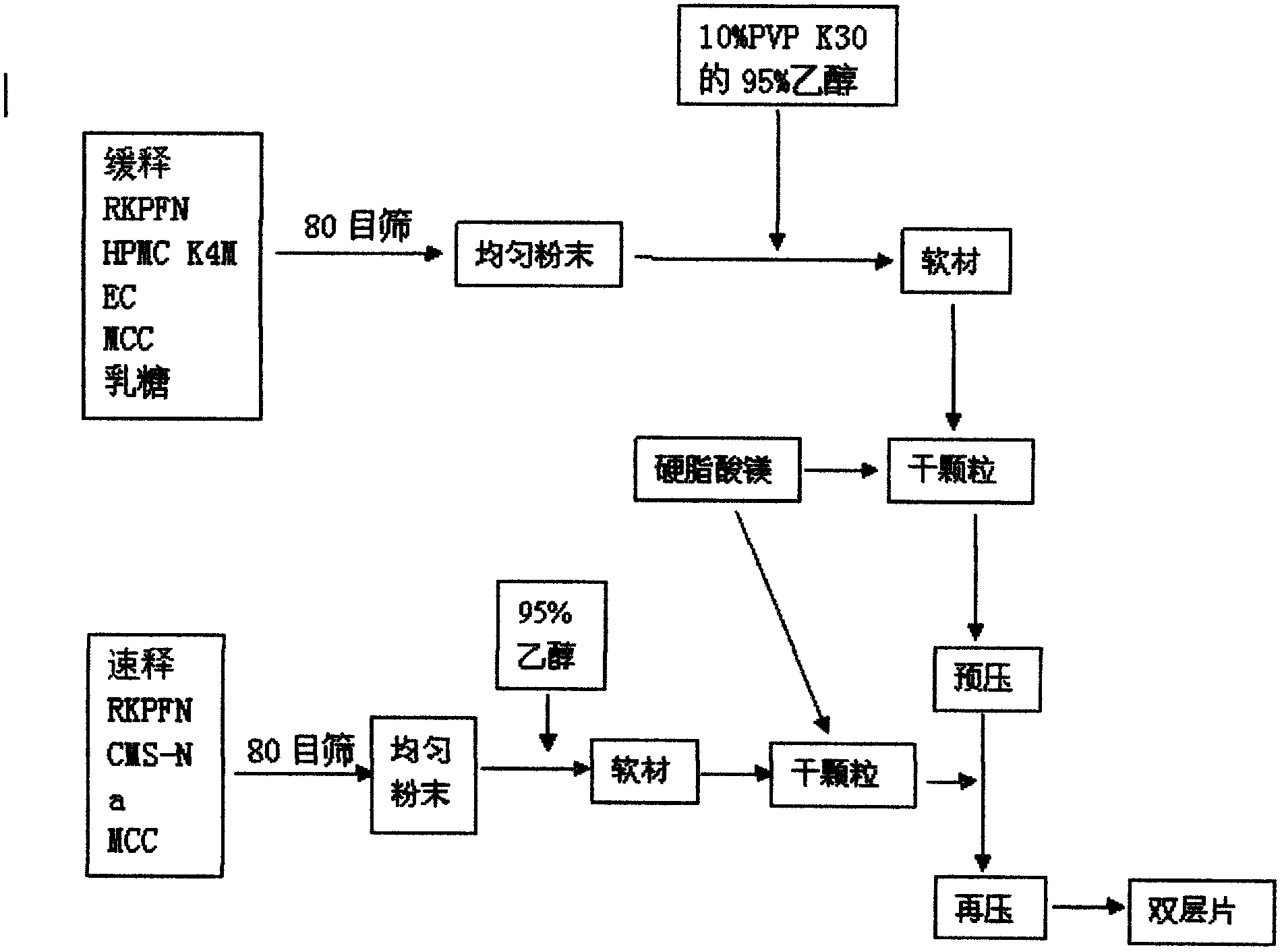
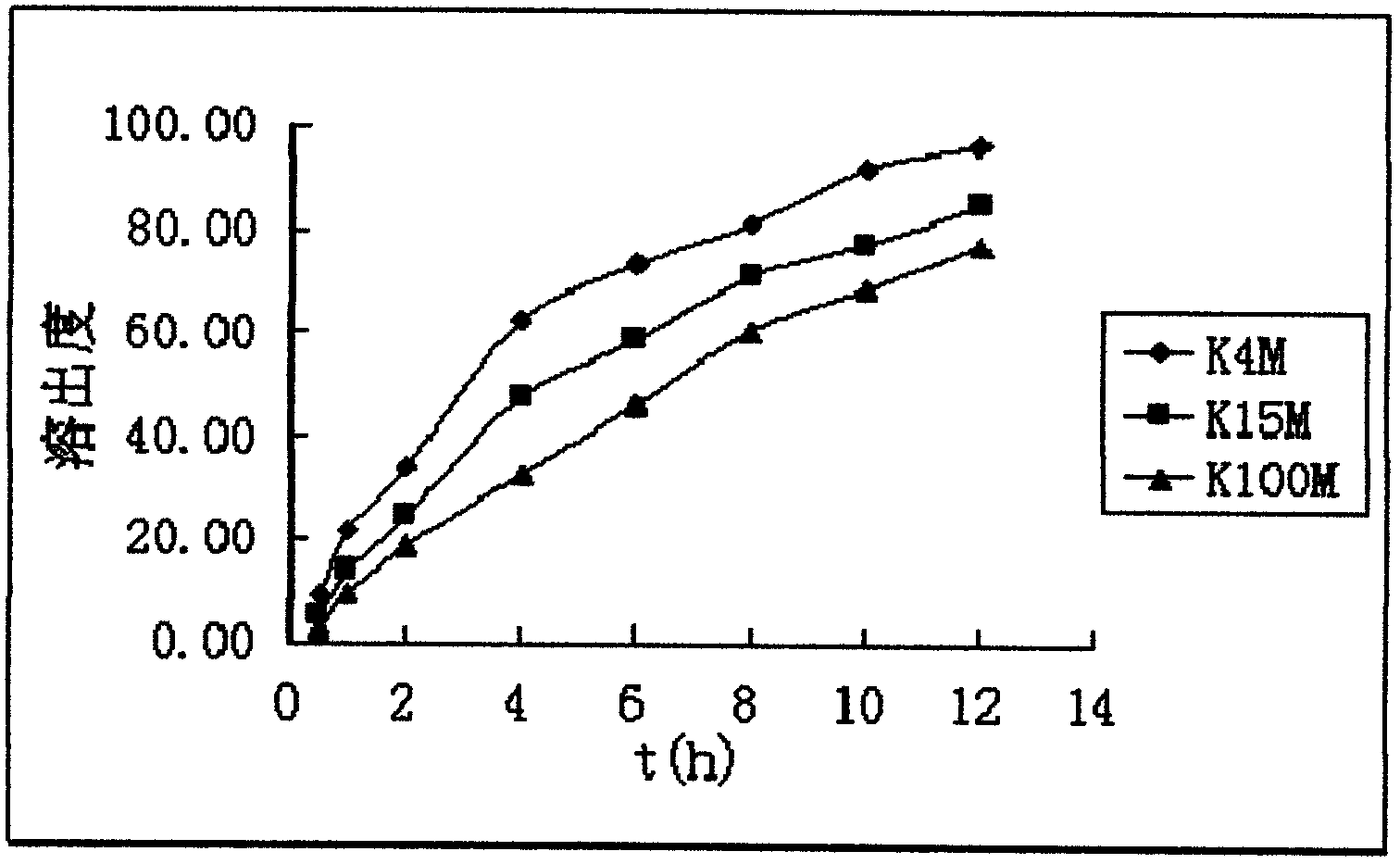
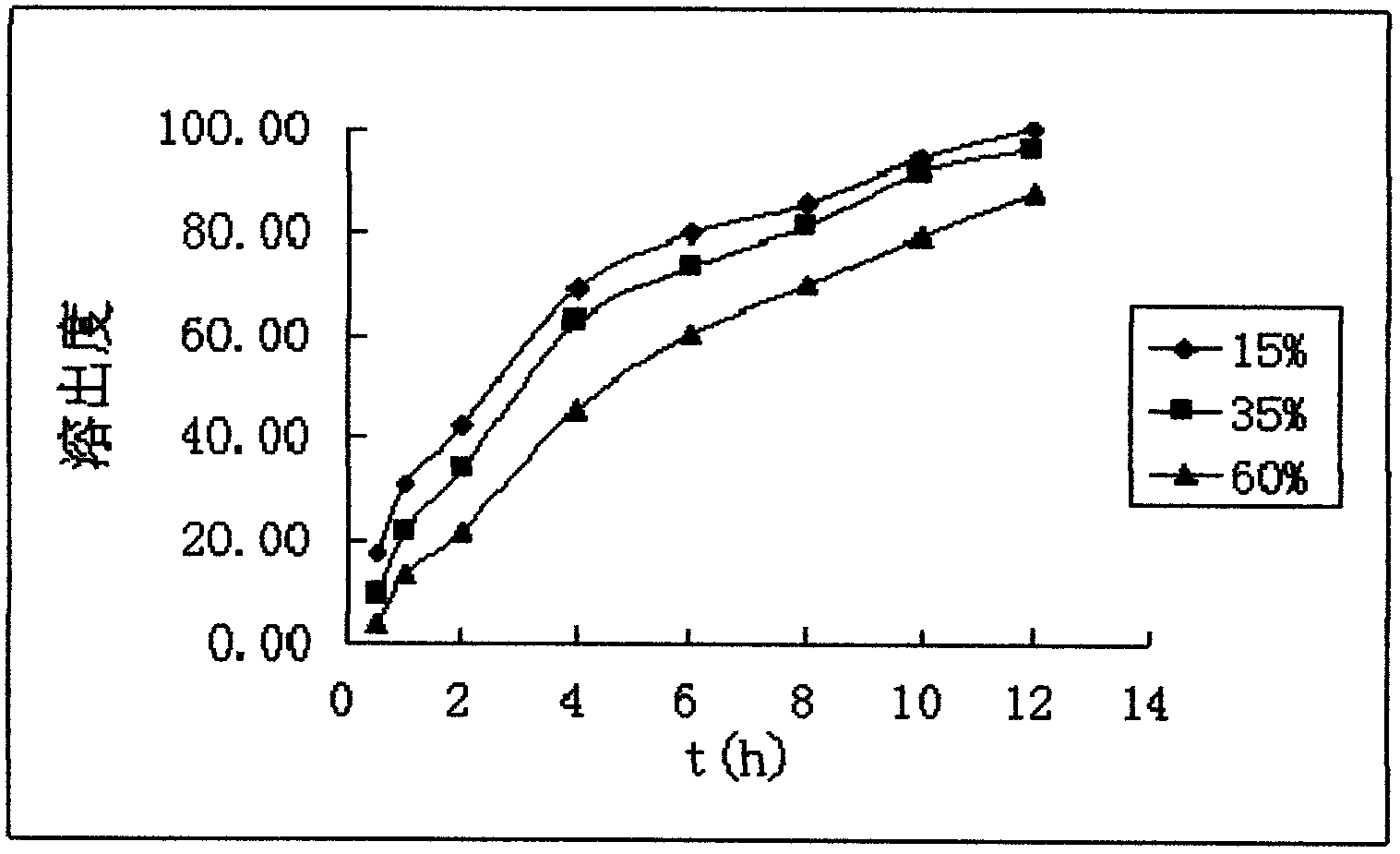
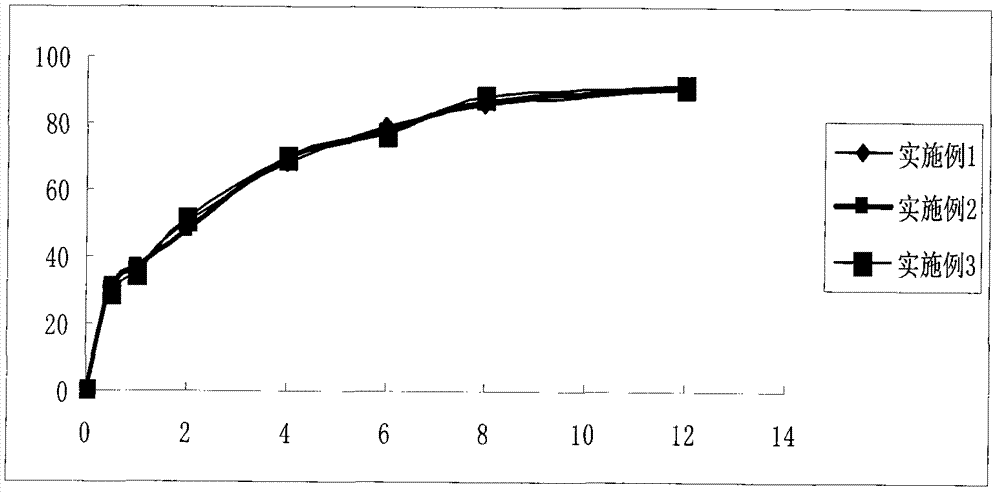
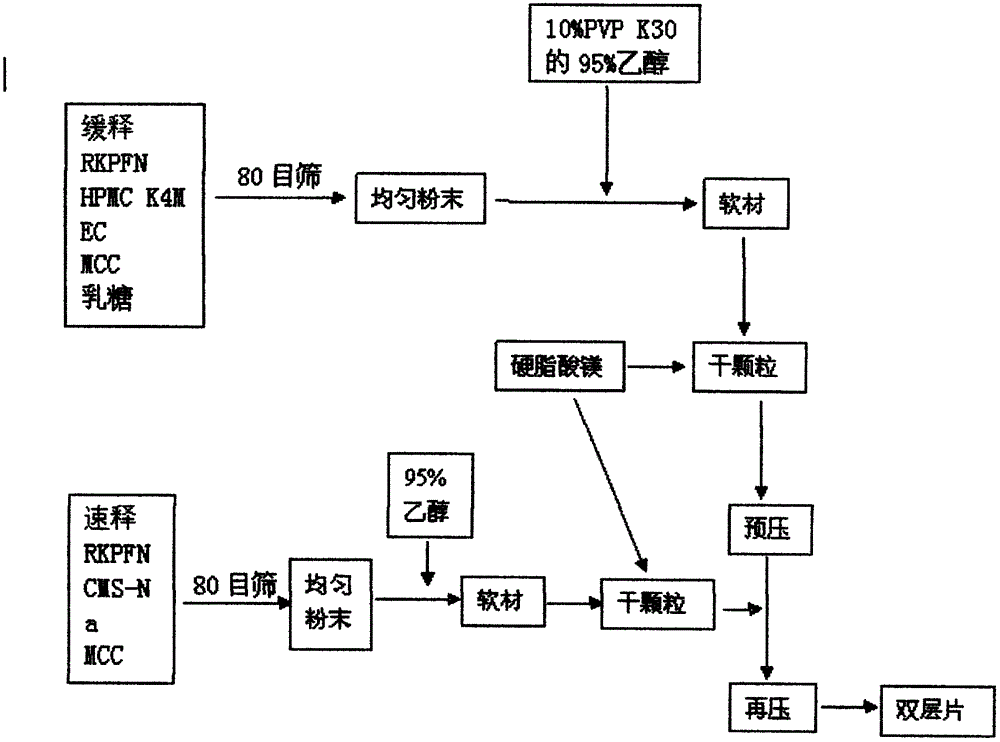
Dexketoprofen trometamol quick-release/sustained-release double-layer tablet and preparation method thereof
ActiveCN103655504AImprove complianceSmooth playbackOrganic active ingredientsAntipyreticBlood concentrationSide effect
The invention relates to a dexketoprofen trometamol quick-release / sustained-release double-layer tablet and a preparation method thereof, and provides a simple and convenient preparation process method for pressing the double-layer tablet consisting of a quick-release layer and a sustained-release layer, wherein the quick-release layer mainly comprises dexketoprofen trometamol, a sustained-release, a diluent, a binding agent and a lubricant. According to the dexketoprofen trometamol quick-release / sustained-release double-layer tablet provided by the invention, due to release of the quick-release part, the medicine is enabled to rapidly achieve effective blood concentration, the sustained-release part can be released slowly, and the effect that the dexketoprofen trometamol can be continuously released for 12 hours when being taken once is achieved. The defects that the conventional dexketoprofen trometamol preparation needs to be taken for multiple times in clinical treatment, and the blood concentration is not stable are made up, so that the effects of improving compliance of patients, stable curative effect and small toxic and side effects are achieved. The tablet is simple in preparation method, raw materials and auxiliaries can be easily obtained, and the tablet is applicable to industrial production and has a good application prospect.
Owner:GUIYANG MEDICAL UNIVERSITY
Preparation method of dexketoprofen trometamol double-layer sustained-release tablets
InactiveCN102813638AImprove complianceSmooth playbackOrganic active ingredientsPeptide/protein ingredientsBlood concentrationPatient compliance
The invention relates to dexketoprofen trometamol double-layer sustained-release tablets and a preparation method thereof. The tablets are characterized in that the tablets are double-layer tablets obtained by compacting a fast-release layer and a sustained-release layer. The fast-release layer is mainly composed of dexketoprofen trometamol, a disintegrating agent, a diluent agent, an adhesive, and a lubricant. The sustained-release layer is mainly composed of dexketoprofen trometamol, a sustained-release material, a diluent agent, an adhesive, and a lubricant. An effective dosage of dexketoprofen trometamol is 10mg-120mg. A main medicine content ratio of the fast-release layer to the sustained-release layer is 1:(1-5). The invention also provides a preparation method of the dexketoprofen trometamol double-layer sustained-release tablets, and a release-degree testing method of the tablets. With the release of the fast-release part of the dexketoprofen trometamol double-layer sustained-release tablets provided by the invention, an effective blood concentration of the medicine can be reached fast. The sustained-release part is released slowly for maintaining a stable and uniform effective blood concentration. Therefore, medication times can be reduced, patient compliance can be improved, the treatment effect is stable, and toxic and side effects are low.
Owner:GUIYANG MEDICAL UNIVERSITY
Dexketoprofen injection and preparation method thereof
ActiveCN102885766ASmall doseLittle side effectsOrganic active ingredientsAntipyreticSolubilitySide effect
The invention discloses a dexketoprofen injection which contains dexketoprofen, an alkaline latent solvent and water for injection, wherein 25g dexketoprofen is contained in the dexketoprofen injection per 1000mL; the molar ratio of the dexketoprofen to the alkaline latent solvent is (1: 0.5)-1.5; and the alkaline latent solvent is alkaline amino acid. In addition, the invention further discloses a preparation method of the injection. In the injection, racemic ketoprofen is replaced by the dexketoprofen, so dosage is little, and side effect is small; and alkaline amino acid is used as a latent solvent, which solves the solubility problem of the dexketoprofen and also overcomes the shortcomings of other latent solvents used in the current preparation; furthermore, the injection can be administered in an intravenous drip way owing to no use of painkiller phenylcarbinol in the injection, thus increasing the medicine administration way; and better safety is ensured because an organic solvent is not used.
Owner:西安远大德天药业股份有限公司
Dexketoprofen coating sustained-release micro-encapsulated capsule
ActiveCN101756939AReduce the frequency of takingSmall fluctuations in blood concentrationOrganic active ingredientsAntipyreticUse medicationTherapeutic effect
The invention provides a dexketoprofen coating sustained-release micro-encapsulated capsule and a preparation method thereof, which belong to the technical filed of chemico-pharmaceutical preparations. The coating sustained-release micro-encapsulated capsule consists of coating sustained-release particles and a capsule shell. The coating sustained-release particles are made of core particles containing dexketoprofen basic remedy through a coating technique. The invention can reduce the stimulation of the dexketoprofen on gastrointestinal tract and taking times, consequently, the medication compliance of patients is improved, the fluctuation of dexketoprofen blood concentration is reduced, bioavailability and treatment effect are improved and adverse reactions are reduced.
Owner:NEW FOUNDER HLDG DEV LLC +2
Dexketoprofen glueosceoctylamine and preparation process thereof and medicine composition having active composition of same
InactiveCN1418886ASimple processHigh split rateOrganic active ingredientsAntipyreticAnkylosing spondylitisDexketoprofen
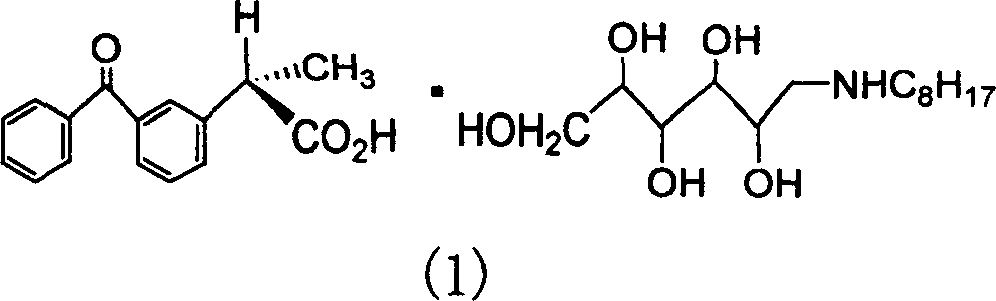
The present invention relates to a dextroketoprofen glucoseoctylamine, its preparation method and medicine composition containing said active component. Its preparation method includes: preparing dextroketoprofen glucoseoctyline salt crude crystal, recrystallization to obtain dextroketoprofen glucoseoctylamine salt and recovering process of (-)-ketoprofen and N-octyl glucosamine. Said compound possesses the actions of clearing away heat, stopping pain and resisting inflammation, its medicine composition can be used for curing rheumatic and rheumatoid arthritis, ankylosing spondylitis, gouty arthritis and various pains.
Owner:NO 1 AUXILARY HOSPITAL NO 4 MILLITARY MEDICAL UNIV P L A
Gel type dexketoprofen plaster and method for preparing the same
InactiveCN100361652CImprove hydrophilicityLittle side effectsOrganic active ingredientsAntipyreticCross-linkMedicine
The invention discloses a gel type dexketoprofen plaster and method for preparation, which comprises backing material, gel type medicament material and anti-sticking material, wherein the medicament material contains 0.5-30.0 wt% of active medicinal constituents, 0.5-40.0 wt% of medicinal solvent, 0.5-30.0 wt% of humectant, and 0.5-8.0 wt% of transderrnal absorption promoting agent, the gel type medicinal material includes high- and low-molecular-weight poly vinyl pyrrolidone or copolymer containing vinyl pyrrolidone unit, polyvinyl alcohol, or polyvinyl alcohol and polyethylene glycol, cross linking agent and water. The invention also discloses its preparation.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Method for preparing dexketoprofen
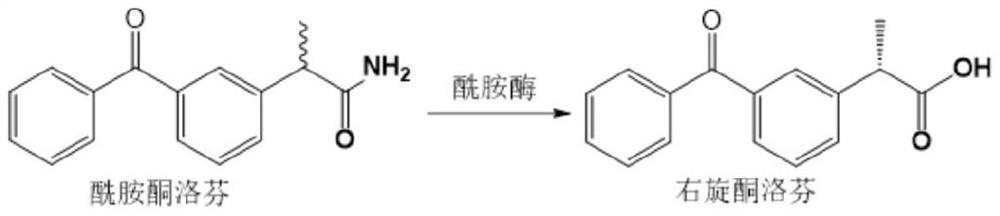
InactiveCN111363736AImprove catalytic reaction efficiencyStrong specificity recognition abilityHydrolasesMicroorganism based processesCombinatorial chemistryAmidase
The invention provides a method for preparing dexketoprofen. The method comprises deamination of ketoprofen amide catalyzed by amidase derived from Klebsiella to obtain dexketoprofen. The inventors found that the amidase derived from Klebsiella has stronger specific recognition ability to dexketoprofen, and the dexketoprofen is prepared by using the amidase derived from Klebsiella, so that the eevalue of dexketoprofen can be greatly.
Owner:HEC PHARM
Dexketoprofen glueosceoctylamine and preparation process thereof and medicine composition having active composition of same
InactiveCN1276925CSimple processHigh split rateOrganic active ingredientsAntipyreticAnkylosing spondylitisClinical study
Owner:NO 1 AUXILARY HOSPITAL NO 4 MILLITARY MEDICAL UNIV P L A
Amidase variant with improved specific activity and application thereof
PendingCN114134132AReduce the amount addedReduce manufacturing costBacteriaHydrolasesEnzyme synthesisEnzyme variant
The invention provides an amidase variant with improved specific activity. The amidase variant is obtained by amidase from Rhodococcus erythropolis MP50 through point mutation, and the amidase variant has the advantages that the specific activity of the amidase variant is improved, and the specific activity of the amidase variant is improved. The invention also relates to application of the enzyme in preparation of dexketoprofen by a biological enzyme catalysis technology. When the amidase variant disclosed by the invention is used for catalytically producing dexketoprofen, the substrate conversion rate is high, and the e.e. Value of a product is gt; and the enzyme input amount is small, so that the cost of producing dexketoprofen by a biological enzyme synthesis method is effectively reduced.
Owner:HEC PHARM
Effervescent formulations comprising dexketoprofen
The present invention relates to water-soluble formulations comprising the active agent dexketoprofen and to a process for production of said formulations. The present invention also relates to pharmaceutical formulations comprising dexketoprofen which is used in symptomatic treatment of mild to moderate pains such as musculoskeletal pains, dysmenorrhoea, toothache, post-operative pains. The formulations are characterized in being in effervescent form.
Owner:BILGIC MAHMUT
Combination Therapies For Treating Metabolic Disorders
InactiveUS20140357602A1Improve the level ofAvoid complicationsBiocideSalicyclic acid active ingredientsCombined Modality TherapyInsulin resistance
Owner:GENMEDICA THERAPEUTICS SL
Compound pharmaceutical composition of tapentadol hydrochloride and dexketoprofen trometamol
InactiveCN104188947AGood analgesic effectSynergisticOrganic active ingredientsNervous disorderAntagonismTapentadol Hydrochloride
The invention relates to a compound pain-relieving pharmaceutical composition which comprise the active ingredients of tapentadol hydrochloride and dexketoprofen trometamol. The invention also relates to a preparation method and application of a compound preparation of the pharmaceutical composition, wherein a dosage form of the compound preparation is an oral solid preparation. Tapentadol hydrochloride and dexketoprofen trometamol have a synergistic effect on the curative effect and antagonism on the toxicity, so that the compound pain-relieving pharmaceutical composition refers to a quick-acting, powerful and low-addiction compound pain-relieving preparation.
Owner:ANHUI YIXINMING PHARMA TECH
Dexketoprofen trometamol gel plaster and preparation method thereof
ActiveCN111789832AGood curative effectLittle side effectsOrganic active ingredientsAntipyreticSacroiliitisDexketoprofen
The invention discloses a dexketoprofen trometamol gel plaster and a preparation method thereof. The dexketoprofen trometamol gel plaster comprises the following components in percentage by weight: 0.1%-3.0% of dexketoprofen trometamol and a gel matrix. According to the dexketoprofen trometamol gel plaster, the prescription of the gel matrix is improved, the plaster does not contain a permeation enhancer, has permeability with high strength, is high in transdermal absorption speed, is capable of being continuously administrated for 24 hours and stable in quality, meets medicine listing requirements, is suitable for various acute and chronic pains such as rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, gouty arthritis, cancer pain, acute sprain, soft tissue contusion pain andthe like, and is high in curative effect, small in side effect, safe and reliable compared with similar products on the market.
Owner:HUNAN JIUDIAN PHARMA
Stable liquid composition of ketoprofen, salts and enantiomers thereof
InactiveUS20200281879A1Improve palatabilityImproved chemical-physicalOrganic active ingredientsDispersion deliveryOral medicationCyclodextrin
A liquid pharmaceutical composition for oral administration comprising a complex of ketoprofen, dexketoprofen or their salts, a β-cyclodextrin and a hydroxyalkylamine, having good palatability and improved chemico-physical and microbiological stability.
Owner:AZIENDE CHIMHE RIUNITE ANGELINI FRANCESCO A C R A F
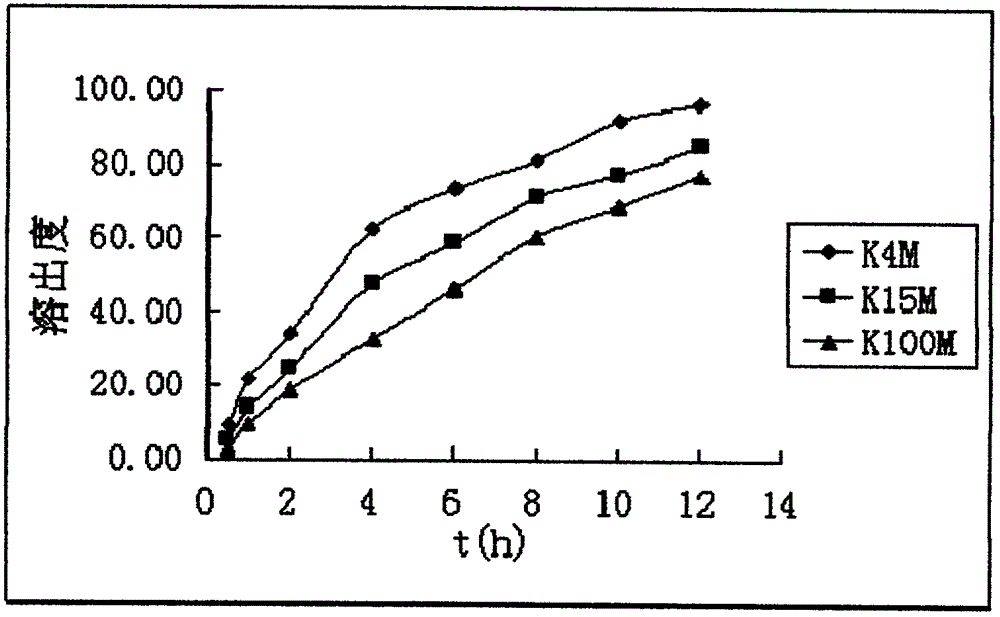
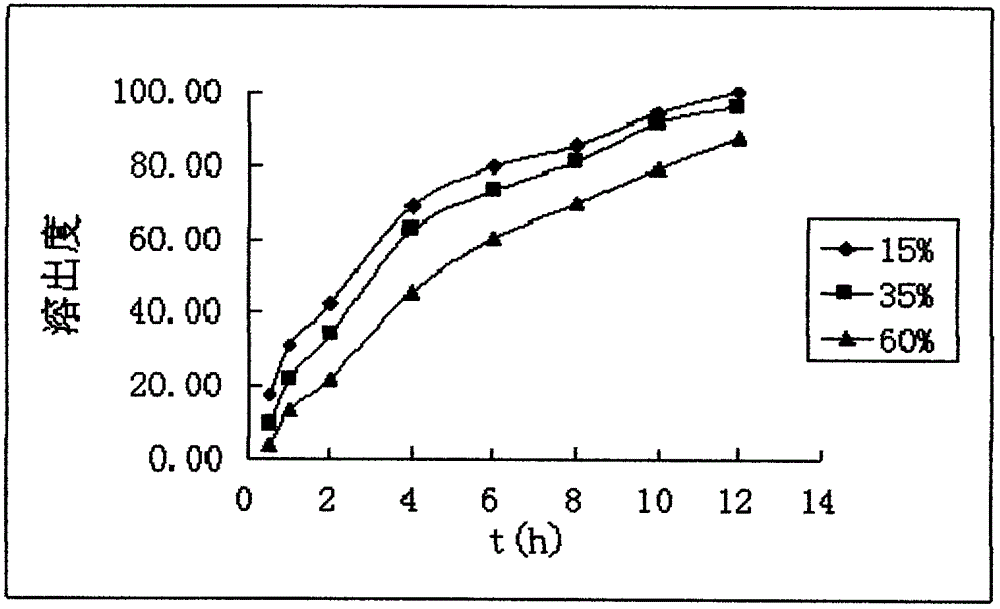
A kind of dexketoprofen tromethamine sustained-release tablet and preparation method thereof
ActiveCN106137997BSmooth releaseThe release medium releases evenly in vitroOrganic active ingredientsAntipyreticDexketoprofen tromethamineProlonged-release tablet
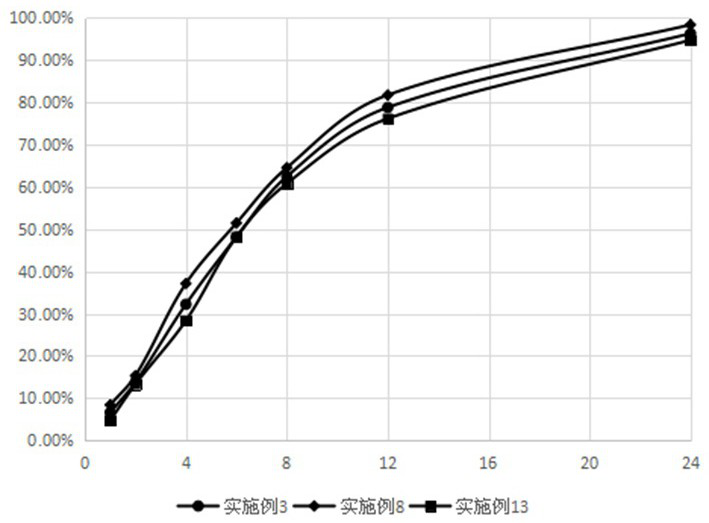
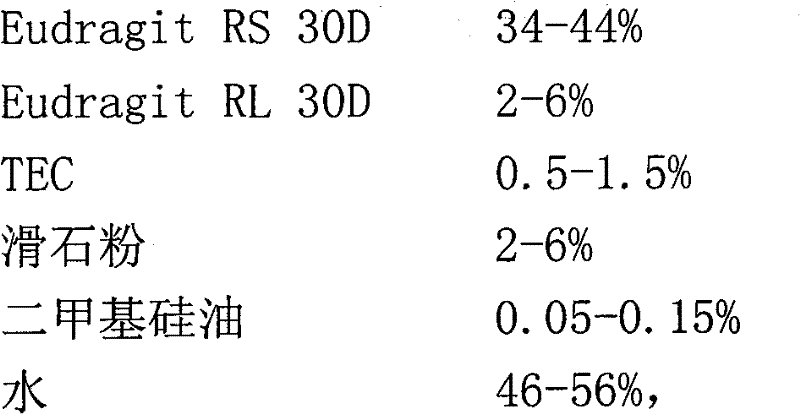
The invention relates to dexketoprofen trometamol sustained-release tablets and a preparation method thereof. The tablets are prepared from a framework material, a lubricant, a flow aid and composition of PC-10 and Eudragit EPO. A direct compression manner is adopted in a technology, the composition comprising fully mixed PC-10 and Eudragit EPO is selected as filler, the condition of sudden release after release stagnation due to limitations of the framework material at different pH values and the solubility of raw materials at different pH values or differences of release speeds in different release media is relieved, and the prepared dexketoprofen trometamol sustained-release tablets have similar release curves in the release media with the different pH values and can be uniformly released. The technology is simple, and the method is suitable for large-scale production.
Owner:瑞博(杭州)医药科技有限公司
Stable liquid composition of ketoprofen, salts and enantiomers thereof
InactiveUS20210260003A9Improve palatabilityImprove stabilityOrganic active ingredientsDispersion deliveryOral medicationCyclodextrin
A liquid pharmaceutical composition for oral administration comprising a complex of ketoprofen, dexketoprofen or their salts, a β-cyclodextrin and a hydroxyalkylamine, having good palatability and improved chemico-physical and microbiological stability.
Owner:AZIENDE CHIMHE RIUNITE ANGELINI FRANCESCO A C R A F
Method for producing a bioactive component-containing nano-composite, and a montmorillonite-based, bioactive component-containing nano-composite
PendingUS20220296525A1Low toxicityIncrease chanceKetone active ingredientsGranular deliveryPolymer scienceMontmorillonite
The present invention relates to a method for producing a bioactive component-containing nanocomposite, specifically a method for producing a montmorillonite-based, bioactive component-containing nanocomposite, as well as to a montmorillonite-based nanocomposite containing a bioactive component, particularly curcumin or dexketoprofen.
Owner:TURKUAZ SAGLIK HIZMETLERI MEDIKAL TEMIZLIK KIMYASAL URUNLER SANAYI & TICARET AS
Gel plaster matrix containing dexketoprofen or pharmaceutical salt thereof and preparation method of gel plaster matrix
ActiveCN111803470AExtended storage timeReduce contentOrganic active ingredientsAntipyreticPolymer scienceActive agent
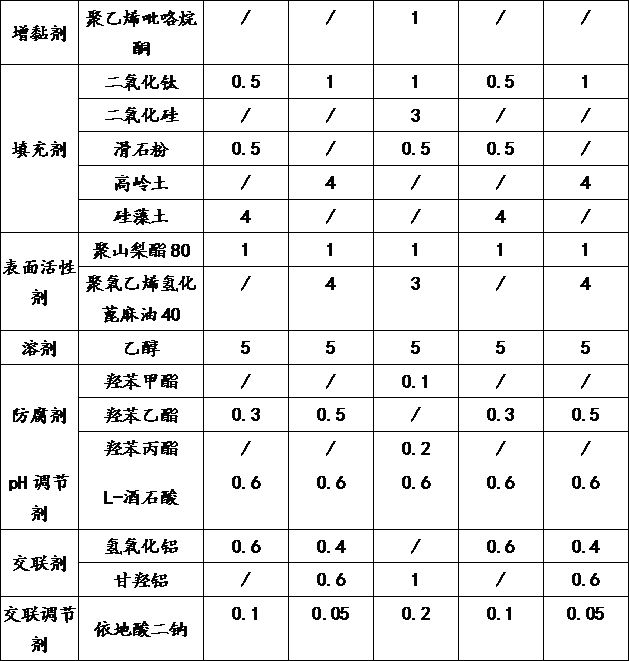
The invention discloses a gel plaster matrix containing dexketoprofen or pharmaceutical salt thereof and a preparation method of the gel plaster matrix. The gel plaster matrix comprises the followingraw materials in percentage by weight: 0.3-1.2% of dexketoprofen or pharmaceutical salt thereof, 5-10% of a polymer hydrophilic gel skeleton material, 20-40% of a humectant, 1-5% of a tackifier, 2-5%of a filler, 0.5-2% of a surfactant, 0.2-1% of a pH regulator, 0.01-0.6% of a cross-linking agent, 0.01-0.2% of a cross-linking regulator and 40-55% of a solvent, wherein the humectant is polyethyleneglycol and sorbitol. According to the invention, a system of the gel plaster matrix is improved, the polyethylene glycol and the sorbitol are combined to serve as the humectant, so that the risk thatrelated substances are increased and the content of the related substances is reduced due to esterification reaction of glycerol serving as a common humectant and acrylic acid non-steroidal anti-inflammatory drugs is reduced, and meanwhile, the moisture retention performance of the gel plaster matrix and the storage stability of the preparation are improved.
Owner:HUNAN JIUDIAN PHARMA +1
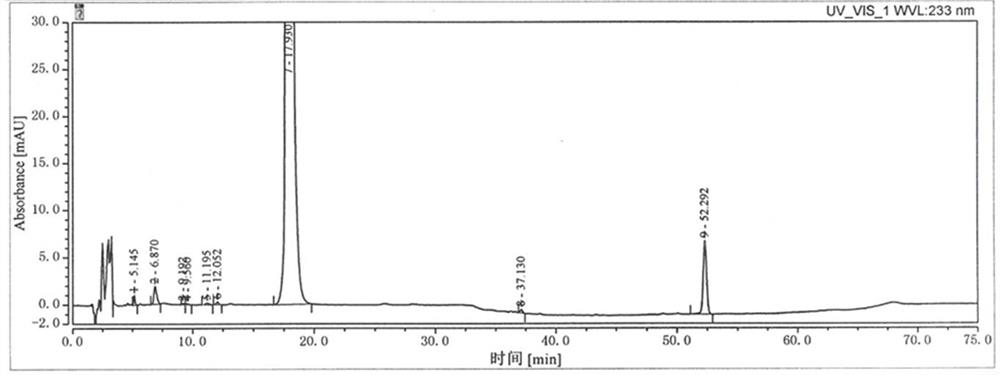
Dexketoprofen trometamol injection and technology for preparing same
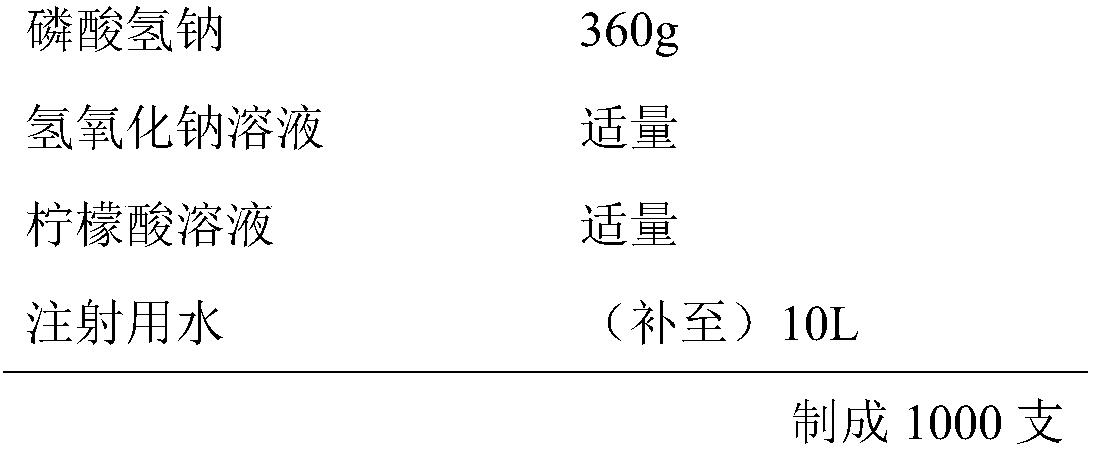
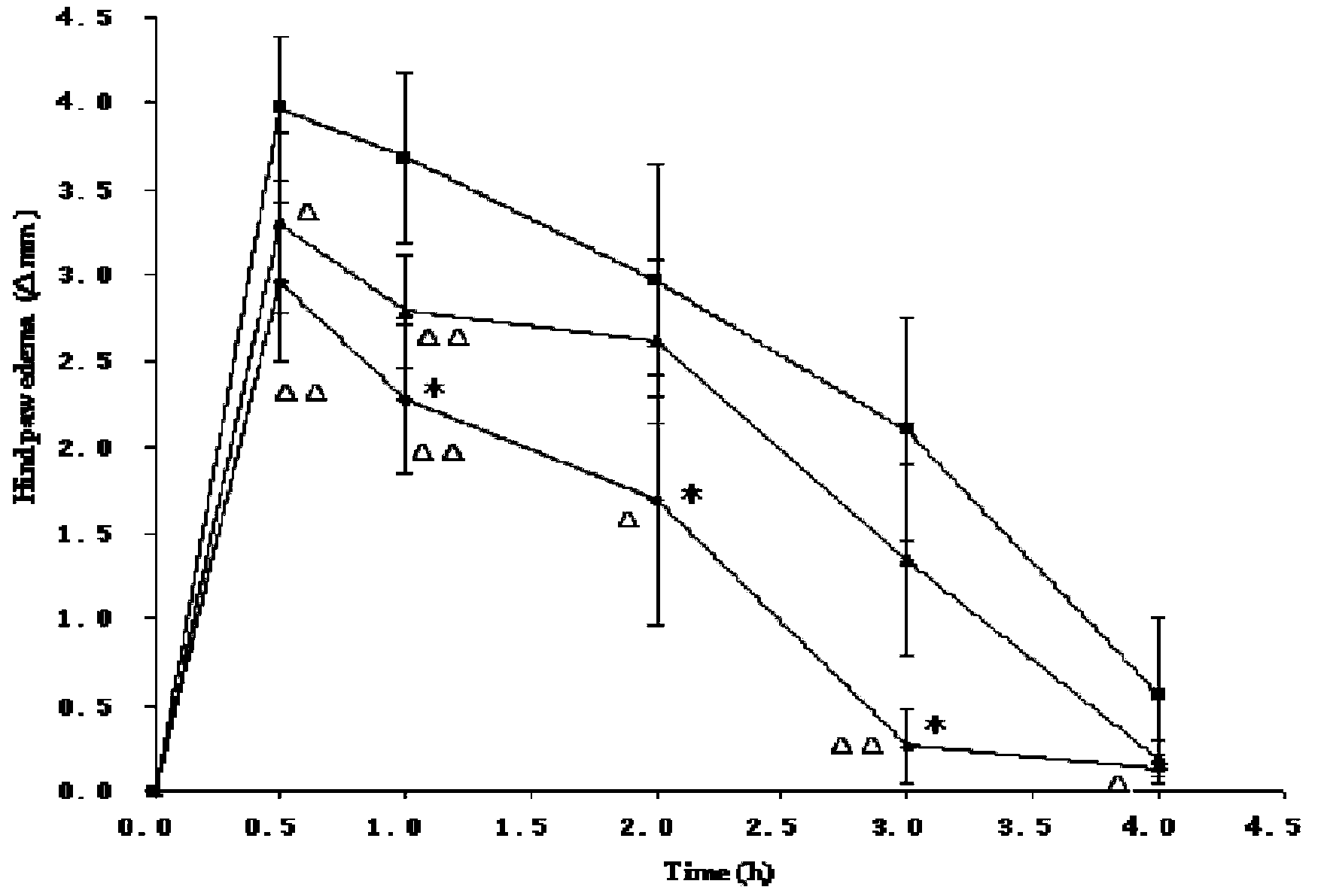
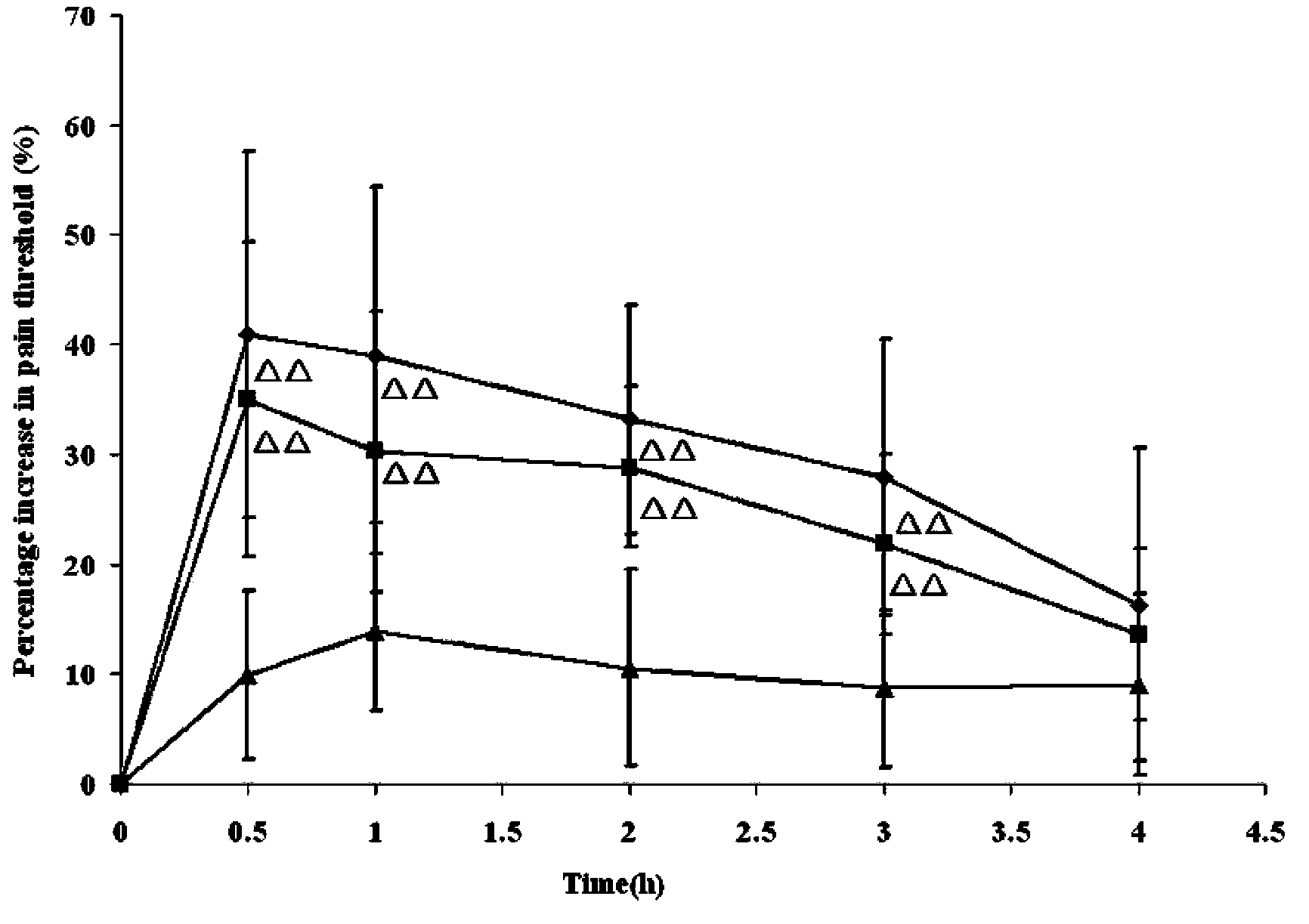
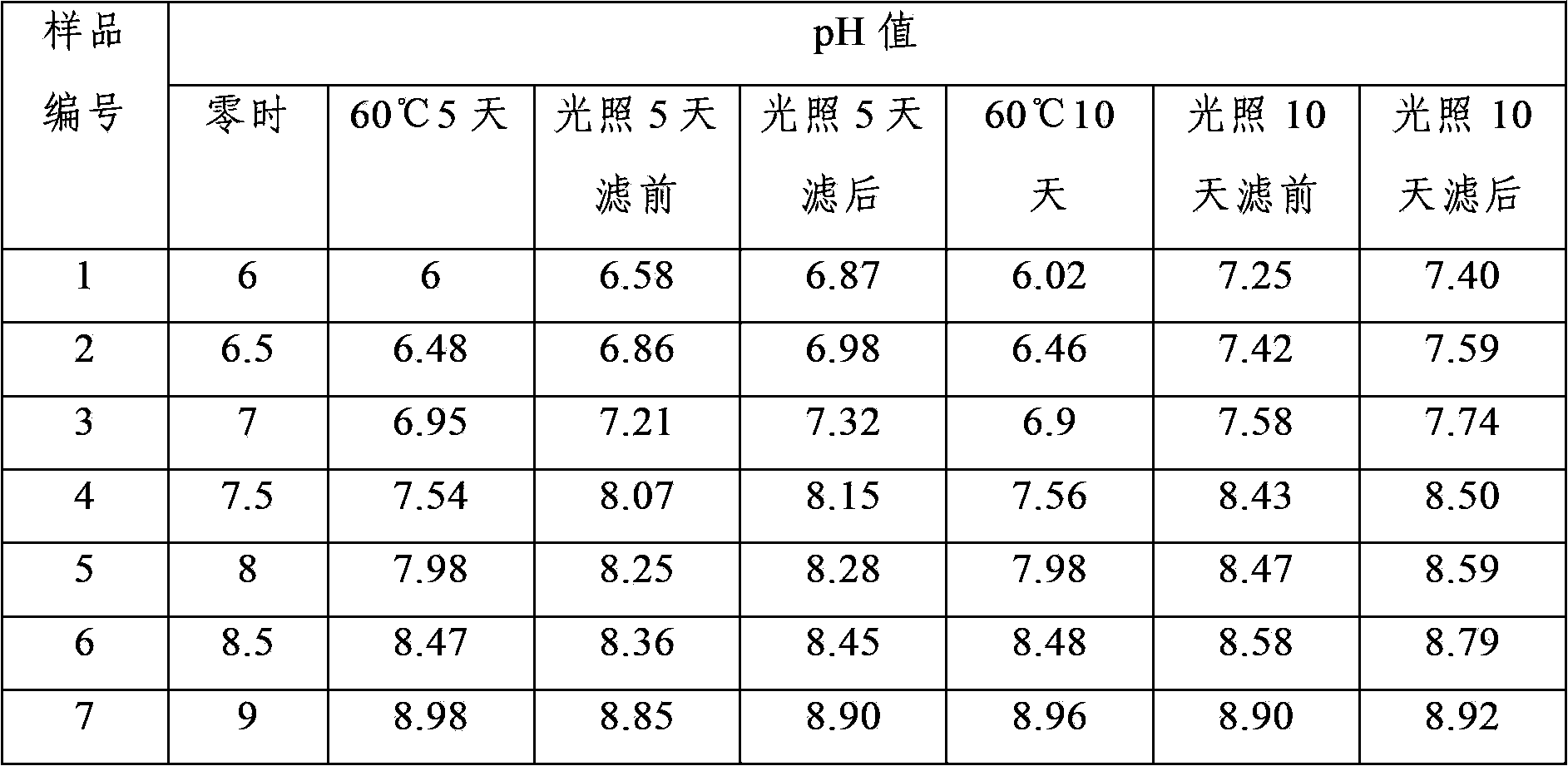
InactiveCN107854429AQuality improvementHigh clarityOrganic active ingredientsAntipyreticPhosphateCLARITY
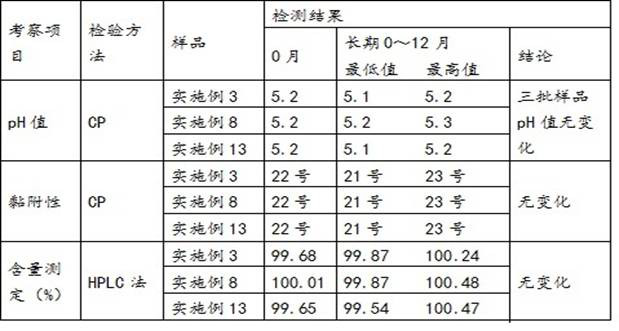
The invention provides dexketoprofen trometamol injection and a technology for preparing the same. The dexketoprofen trometamol injection and the technology have the advantages that phosphate is usedas a stabilizer, accordingly, the problem that effective components are easy to degrade in common dexketoprofen trometamol injection production procedures can be solved by the aid of the dexketoprofentrometamol injection and the technology, the dexketoprofen trometamol injection is stable in quality, good in clarity, easy and convenient to operate and suitable for industrial mass production, andtechnological processes are simple.
Owner:NANJING ZENKOM PHARMA
Dexketoprofen injection and preparation method thereof
ActiveCN102885766BSmall doseLittle side effectsOrganic active ingredientsAntipyreticSolubilityOrganic solvent
The invention discloses a dexketoprofen injection which contains dexketoprofen, an alkaline latent solvent and water for injection, wherein 25g dexketoprofen is contained in the dexketoprofen injection per 1000mL; the molar ratio of the dexketoprofen to the alkaline latent solvent is (1: 0.5)-1.5; and the alkaline latent solvent is alkaline amino acid. In addition, the invention further discloses a preparation method of the injection. In the injection, racemic ketoprofen is replaced by the dexketoprofen, so dosage is little, and side effect is small; and alkaline amino acid is used as a latent solvent, which solves the solubility problem of the dexketoprofen and also overcomes the shortcomings of other latent solvents used in the current preparation; furthermore, the injection can be administered in an intravenous drip way owing to no use of painkiller phenylcarbinol in the injection, thus increasing the medicine administration way; and better safety is ensured because an organic solvent is not used.
Owner:西安远大德天药业股份有限公司
Dexketoprofen tromethamine gel patch and preparation method thereof
ActiveCN111789832BGood curative effectLittle side effectsOrganic active ingredientsAntipyreticSpondarthritisSide effect
The invention discloses a dexketoprofen tromethamine gel plaster and a preparation method thereof. The gel plaster contains 0.1% to 3.0% dexketoprofen tromethamine by weight percentage and gel matrix. The dexketoprofen trometamol gel plaster of the present invention is improved through the prescription of the gel matrix, does not contain a penetration enhancer, has high permeability, fast transdermal absorption, can be administered continuously for 24 hours, and has stable quality , in line with drug marketing requirements, suitable for rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, gouty arthritis, and various acute and chronic pains such as cancer pain, acute sprain, soft tissue contusion pain, etc. The product has high curative effect, less side effects, and is safe and reliable.
Owner:HUNAN JIUDIAN PHARMA
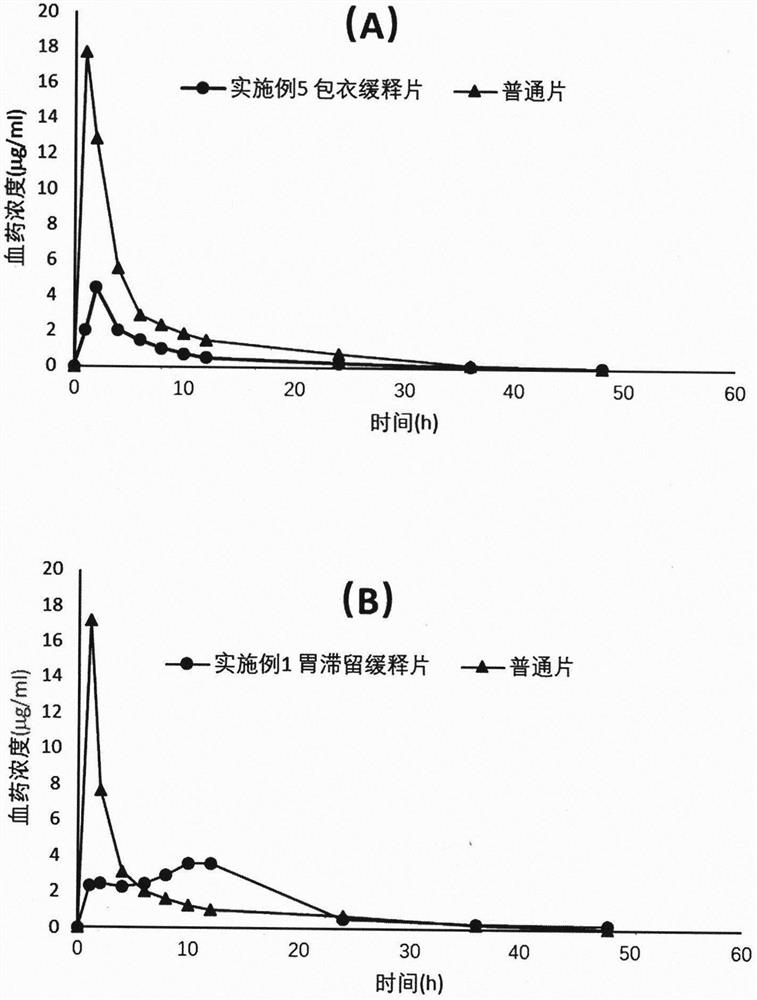
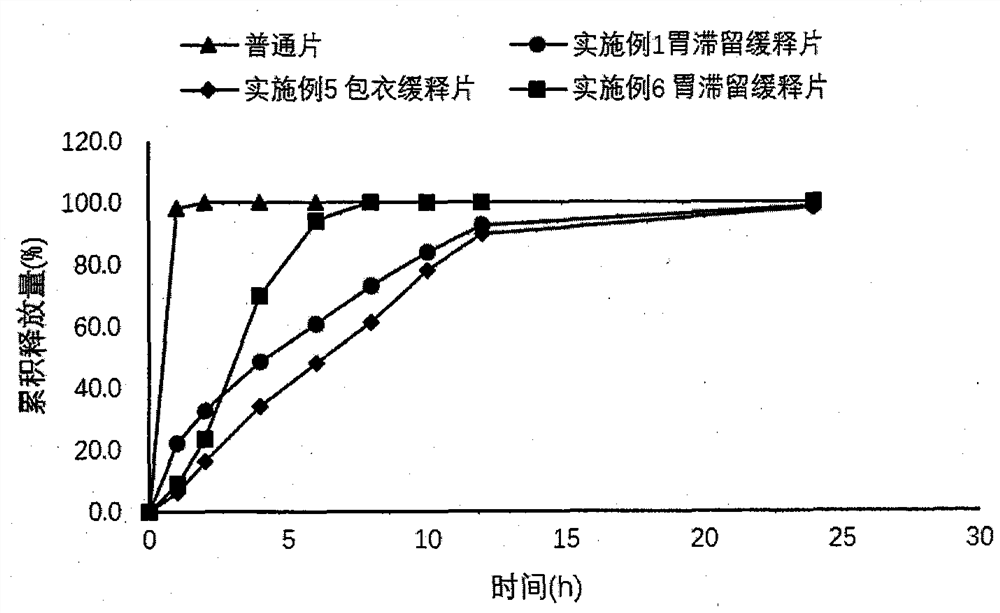
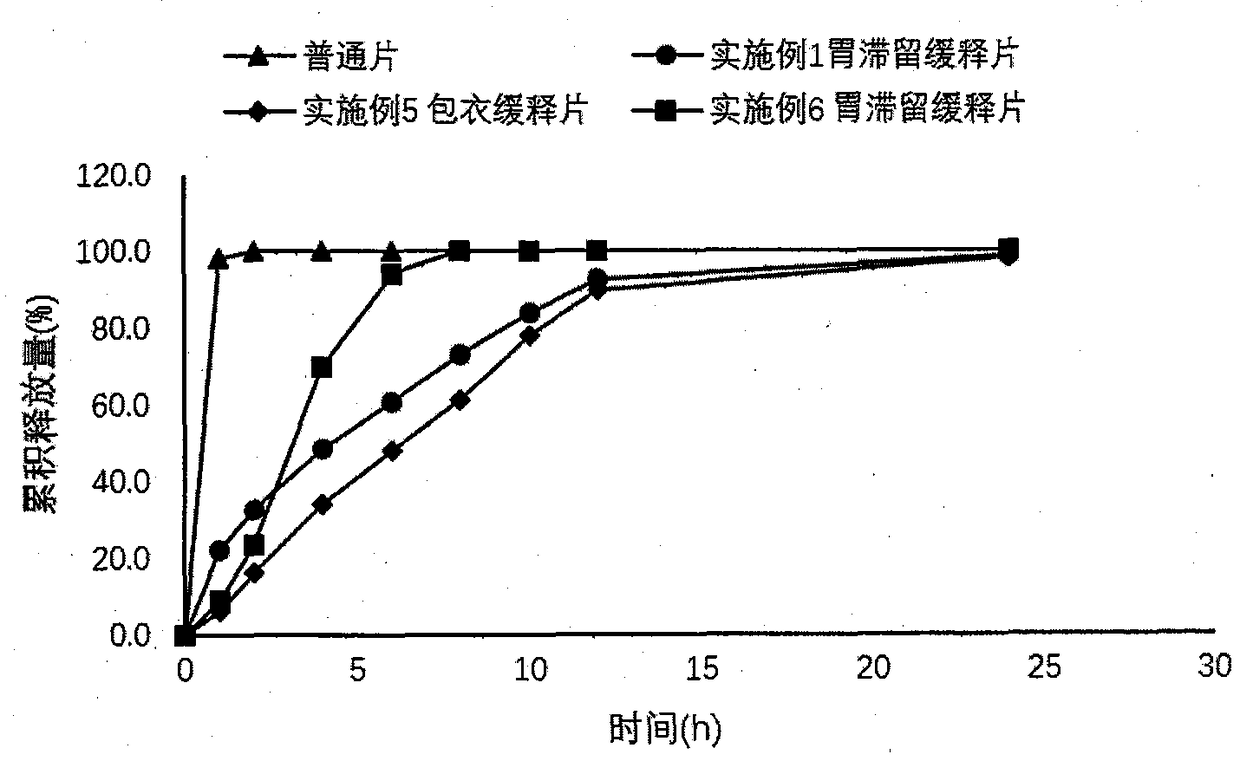
Dexketoprofen Tromethamine Sustained Release Tablets for Gastric Retention
ActiveCN108542889BControlled release rateSmall fluctuations in concentrationOrganic active ingredientsAntipyreticDrug release rateAntiinflammatory drug
The invention provides a dexketoprofen trometamol gastroretentive sustained-release tablet and a preparation method thereof, and belongs to the field of medicines. Dexketoprofen trometamol is a non-steroidal analgesic and anti-inflammatory drug. Ordinary tablets of dexketoprofen trometamol need to be administered frequently, and taking of 1-2 tablets of the ordinary tablets 3-4 times a day is inconvenient for patients. The dexketoprofen trometamol gastroretentive sustained-release tablet can fully absorb gastric juice after being orally taken, and expands to form a hydrogel, so the drug release rate is controlled, the water absorbing expanded hydrogel preparation generates a gastroretentive effect by the comprehensive action of floatation, mucous membrane adherence and the expansion volume, and has a continuous sustained release effect for 24 h in vivo and in vitro; the dexketoprofen trometamol gastroretentive sustained-release tablet can be well absorbed in beagle dogs, reduces the plasma concentration change, achieves one dose per day, and increases the administration compliance of patient; and the gastroretentive sustained-release tablet is prepared by a direct powder tabletingprocess, so the method has the advantages of simple technology, and facilitation of industrial production.
Owner:CHINA PHARM UNIV
Effervescent formulations comprising dexketoprofen
Owner:BILGIC MAHMUT
Dexketoprofen trometamol gastroretentive sustained-release tablet
ActiveCN108542889AControlled release rateSmall fluctuations in concentrationOrganic active ingredientsAntipyreticDrug release rateSustained Release Tablet
The invention provides a dexketoprofen trometamol gastroretentive sustained-release tablet and a preparation method thereof, and belongs to the field of medicines. Dexketoprofen trometamol is a non-steroidal analgesic and anti-inflammatory drug. Ordinary tablets of dexketoprofen trometamol need to be administered frequently, and taking of 1-2 tablets of the ordinary tablets 3-4 times a day is inconvenient for patients. The dexketoprofen trometamol gastroretentive sustained-release tablet can fully absorb gastric juice after being orally taken, and expands to form a hydrogel, so the drug release rate is controlled, the water absorbing expanded hydrogel preparation generates a gastroretentive effect by the comprehensive action of floatation, mucous membrane adherence and the expansion volume, and has a continuous sustained release effect for 24 h in vivo and in vitro; the dexketoprofen trometamol gastroretentive sustained-release tablet can be well absorbed in beagle dogs, reduces the plasma concentration change, achieves one dose per day, and increases the administration compliance of patient; and the gastroretentive sustained-release tablet is prepared by a direct powder tabletingprocess, so the method has the advantages of simple technology, and facilitation of industrial production.
Owner:CHINA PHARM UNIV
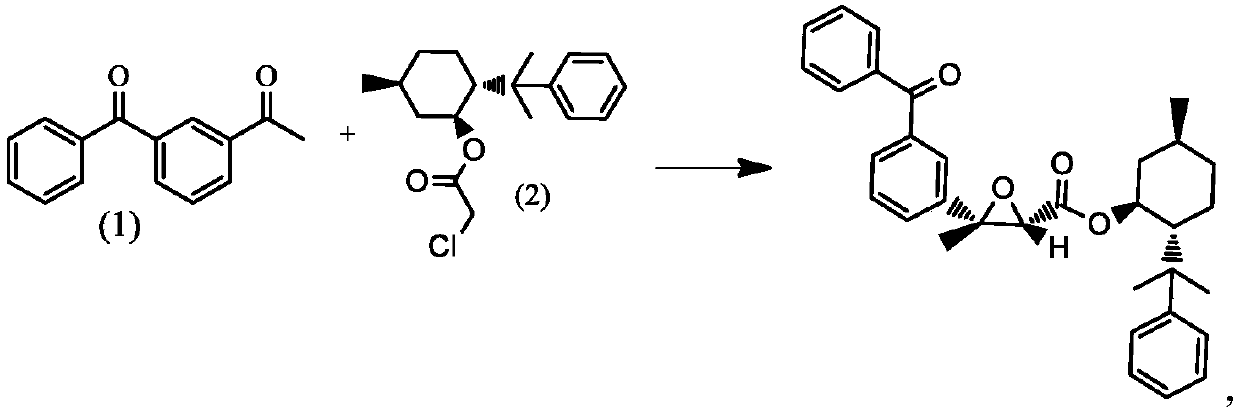
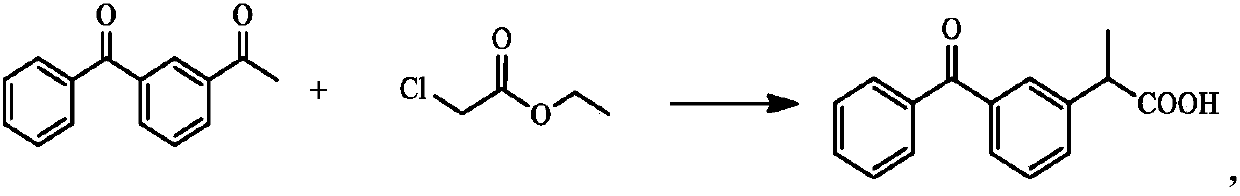
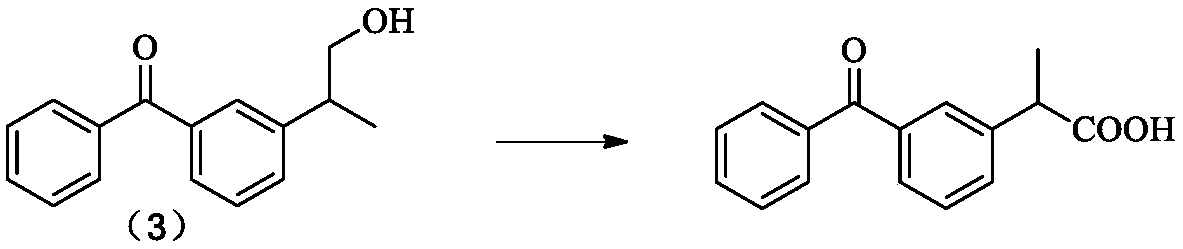
Method for synthesizing dexketoprofen intermediate
The invention belongs to the technical field of medicines, and particularly relates to a method for synthesizing a dexketoprofen intermediate. The dexketoprofen intermediate is prepared by asymmetricsynthesis of Darzens reaction. The method can improve the selectivity of the reaction, reduce the loss of raw materials, improve the yield and simplify the operation process, and is conducive to industrial production.
Owner:NANJING ZENKOM PHARMA
A kind of dexketoprofen coated sustained-release microparticle capsule
Owner:NEW FOUNDER HLDG DEV LLC +2
Gel plaster matrix containing dexketoprofen or its medicinal salt and preparation method thereof
ActiveCN111803470BExtended storage timeReduce contentOrganic active ingredientsAntipyreticPolymer scienceActive agent
The invention discloses a gel plaster matrix containing dexketoprofen or a medicinal salt thereof and a preparation method thereof. The gel plaster matrix, by weight percentage, comprises: dexketoprofen or a medicinal salt thereof 0.3 ~1.2%, polymer hydrophilic gel skeleton material 5~10%, humectant 20~40%, thickener 1~5%, filler 2~5%, surfactant 0.5~2%, pH regulator 0.2~1%, crosslinking agent 0.01~0.6%, crosslinking regulator 0.01~0.2%, solvent 40~55%, among them, the moisturizing agent is polyethylene glycol and sorbitol. In the present invention, by improving the matrix system of the gel plaster, polyethylene glycol and sorbitol are combined as a moisturizer, which reduces the increase of related substances and the The risk of content decline, while improving the moisturizing performance of the gel patch base and the storage stability of the formulation.
Owner:HUNAN JIUDIAN PHARMA +1
Dexketoprofen trometamol quick-release sustained-release double-layer tablet and its preparation process
ActiveCN103655504BImprove complianceSmooth playbackOrganic active ingredientsAntipyreticSide effectBlood concentration
The invention relates to a dexketoprofen trometamol quick-release / sustained-release double-layer tablet and a preparation method thereof, and provides a simple and convenient preparation process method for pressing the double-layer tablet consisting of a quick-release layer and a sustained-release layer, wherein the quick-release layer mainly comprises dexketoprofen trometamol, a sustained-release, a diluent, a binding agent and a lubricant. According to the dexketoprofen trometamol quick-release / sustained-release double-layer tablet provided by the invention, due to release of the quick-release part, the medicine is enabled to rapidly achieve effective blood concentration, the sustained-release part can be released slowly, and the effect that the dexketoprofen trometamol can be continuously released for 12 hours when being taken once is achieved. The defects that the conventional dexketoprofen trometamol preparation needs to be taken for multiple times in clinical treatment, and the blood concentration is not stable are made up, so that the effects of improving compliance of patients, stable curative effect and small toxic and side effects are achieved. The tablet is simple in preparation method, raw materials and auxiliaries can be easily obtained, and the tablet is applicable to industrial production and has a good application prospect.
Owner:GUIYANG MEDICAL UNIVERSITY
Dexketoprofen tromethamine capsule and preparation process thereof
InactiveCN112603902AQuick effectGood analgesic effectOrganic active ingredientsNervous disorderDexketoprofen tromethamineMethyl cellulose
The invention discloses a dexketoprofen tromethamine capsule which is characterized by being prepared from the following components, by weight: 3.7 parts of dexketoprofen tromethamine, 6 parts of pregelatinized starch, 14 parts of microcrystalline cellulose, 1 part of hydroxypropyl methyl cellulose, a proper amount of magnesium stearate, a proper amount of talcum powder and a proper amount of 30% ethanol. The preparation method comprises the following steps of mixing, granulating, granule straightening, total mixing, sampling and inspecting to obtain a finished product. Compared with the prior art, the dexketoprofen tromethamine capsule has the advantages of quick response, better analgesic effect and small side effect.
Owner:BEIJING ASIA EAST BIO PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com